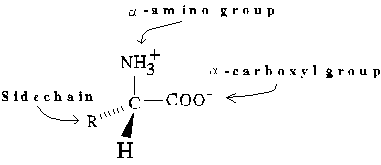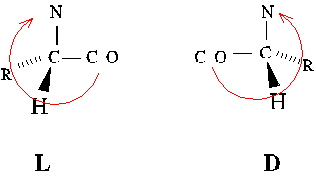Biochemistry 601
September 1, 1999 Amino Acids of Naturally Occurring Proteins
Last modified 9/1/99.
Dr. Landry
Rm. 6055
landry@mailhost.tcs.tulane.edu
Reading Assignment
-
Voet, Voet, and Pratt (VVP), Chapter 2, "Water", pp. 22-37
-
VVP, Chapter 4, "Amino Acids", pp. 77-91
Resources
Topics for discussion
-
Chemical structure and properties of amino acids.
-
Hierarchy and features of protein 3-dimensional structure.
-
Mechanism of formation and stability of protein structure.
-
Common structural themes in the expression of protein function.
Proteins have diverse functions: structural, enzymatic, regulatory.
For example consider the following diseases and their associated defective
proteins:
DISEASE DEFECTIVE PROTEIN VVP, p.
Genetic Defects
cystic fibrosis Cl- channel
familial hypercholesterolemia LDL receptor 264
hemolytic anemia G-6-P dehydrogenase
hemophilia (VIII) factor X protease activator 318
I-cell disease UDP-GlcNAc phosphotransferase 260
sickle cell anemia hemoglobin 176
xeroderma pigmentosum DNA exinuclease 799
cancer ras, tyrosine kinase, etc. 682
Marfan syndrome fibrilin
Dietary Deficiencies
scurvy (ascorbate) collagen 135
beriberi (thiamine) pyruvate dehydrogenase, 404
a-ketogluterate dehydrogenase,
pyruvate dehydrogenase
Amyloidoses
Alzheimer's disease amyloid protein precursor (APP) 156
Prion diseases prion protein precursor (PrP) 156
Diverse functions are expressed in the 3-dimensional structure of
proteins in the form of catalytic sites, regulated ligand binding sites,
fiber-like properties, etc.
All proteins are linear chains of amino acids linked together by peptide
bonds to form polypeptides. Protein 3-dimensional structure
is specified by the linear sequence of amino acids. Noncovalent interactions
within the polypeptide and between the polypeptide and solvent (water)
direct the formation and stability of the native protein fold.
Amino Acids - Structure and Properties
Reading assignment:
Objectives
-
Review ionization
of weak acids and bases
-
Review structure and classification
of the twenty amino acids that occur in proteins synthesized by ribosomes.
-
See that proteins are built by linking amino acids together with peptide
bonds.
-
Recognize that post-translational
modification of amino acids can confer additional chemical and physical
properties to polypeptides.
Ionization of Weak Acids and Bases (VVP, pp. 31-36)
Given an acid (HA) that dissociates into a proton (H+) and the conjugate
base (A-), we express the equilibrium dissociation as
Ka = [H+][A-]/[HA] ; and rearrange as
1/[H+] = (1/Ka)([A-]/[HA])
Assume:
pH = - log [H+] = log (1/[H+])
pK = - log [Ka] = log (1/Ka)
substitute to obtain the Henderson-Hasselbalch equation:
pH = pKa + log ([A-]/[HA]) [1]
Example 1
Determine the carboxylate/carboxylic acid ratio for the alpha-carboxyl
group of alanine (pKa = 2.3) in solution at neutral pH.
Substituting into equation 1:
7 = 2.3 + log ([A-]/[HA]) ; subtract 2.3 from both sides...
[A-]/[HA] = 10**4.6 , which is more than 10,000/1
Example 2
At pH =9.4, what percentage of the lysine sidechain amino group (pK = 10.0)
is uncharged?
9.4 = 10.0 + log (uncharged/cation)
log (uncharged/cation) = -0.6
(uncharged/cation) = 10**(-0.6) = 0.25
Thus, the fraction uncharged is 0.25/(1+0.25) = 0.2 or 20%.
Isoelectric Point (pI)
The Isoelectric Point (pI) is the pH at which a molecule has no net charge.
Amino acids form dipolar ions or zwitterions: 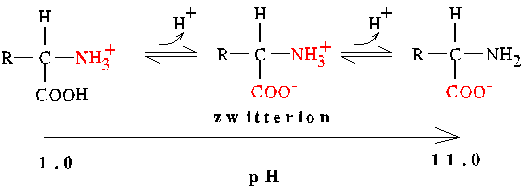
For example, the pI for alanine is evaluated as follows:
pI = 1/2 (pK1 + pK2)
pIAla = 1/2(2.3 + 9.9) = 6.1
The pI of molecules with more than two pK's
For a molecule having more than two ionizable groups, the pI is approximately
equal to the average of the relevant pair of pK's. For example,
aspartic acid has three pK's with the following values:
corresponding to the equilibria shown 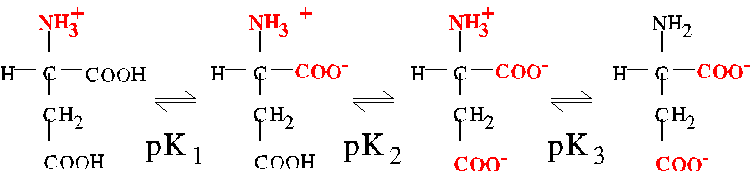
The relevant pair of pK's are pK1 and pK2. At the pH corresponding
to the average of these two pK's, the amino group is fully ionized, and
the two carboxylates are partially ionized. Thus, the pI of aspartate is
as follows:
pIAsp = 1/2(2.0 + 3.9) = 3.0
Sample Problems
-
What is the molarity of pure water? Show that a change in the concentration
of water by ionization does not appreciably affect the molarity of the
solution.
-
When sufficient acid is added to lower the pH by one unit, what is the
corresponding increase in hydrogen ion concentration?
-
You have a solution of HCl that has a pH of 2.1. what is the concentration
of HCl need to make this solution?
-
The charged form of the imidazole ring of histidine is believed to participate
in a reaction catalyzed by an enzyme. At pH 7.0, what is the probability
that the imidazole ring will be charged?
-
Calculate the pH at which a solution of cysteine would have no net charge.
Answers:
1. Water molarity=1000/18=55.6M. Since Kw=1.0x10**(-14), the concentration
of OH(-) and H(+) are 10**(-7) each. Thus, the actual concentration of
water is 55.6-0.0000001. This difference is negligible.
2. Tenfold change in H(+) concentration.
3. Assume that HCl in solution is completely ionized to H(+) and Cl(-).
pH=-log[H(+)]=2.1
[H(+)]=10**(-2.1)=7.94x10**(-3)M
4. For the histidine ring, the pK=6.0.
pH=pK + log ([His]/[His(+)])
7.0=6.0+ log ([His]/[His(+)])
log ([His]/[His(+)])=1.0
([His]/[His(+)])=10
Thus, the ratio is 10:1 and the probability is 1/(10+1)=0.09 or 9%.
5. Average the pK's of the carboxyl (pK=1.8) and sidechain (pK=8.3).
The pI=5.1.
The Twenty Amino Acids
-occuring in proteins translated on ribosomes
View the structures in a kinemage.
Amino acids are important as:
-
precursors for protein synthesis
-
intermediates in metabolism, e.g., ornithine for nitrogen metabolism
and S-adenosyl-methionine for biological methylation
-
neurotransmitters, e.g., GABA, glycine, L-dopamine
-
precursors of other non-protein compounds, e.g., heme, nucleotide bases,
thyroxin, histamine
General form of an L-Amino Acid (zwitterionic form) 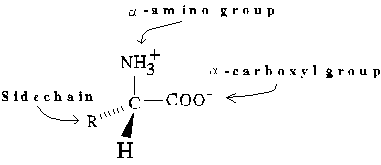
Alternate absolute configurations of amino acids can be distinguished
using the "corncrib" mnemonic. Note the order of alpha-carbon substituents,
CO-R-N, is clockwise in the L configuration. 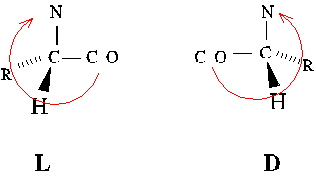
D-amino acids are used by bacteria and plants. They are incorporated
into polypeptides by specialized ligases.
Amino Acid Classification
Amino acids are grouped by the chemical properties of the sidechain. The
same amino acid can fall into multiple groups.
size - for example, affecting how well the sidechain fits in
a binding site
hydrophobicity- for example, determining whether the sidechain
will be buried in a protein interior or membrane as opposed to interacting
with the solvent (water)
H-bonding potential - for example, providing geometrically specific
interactions or chemical catalysis
charge - for example, providing for long-range electrostatic
interactions
polarity - providing water solubility
chemical structure - i.e., aliphatic, aromatic, sulfur-containing,
aliphatic hydroxyl-containing, basic, acidic, amide derivative
Aliphatic Amino Acids
glycine
alanine
hydrophobic
valine - beta-branched
leucine
isoleucine - beta-branched, second chiral center
a-imino acid
proline - containing a pyrrolidine ring
Aromatic Amino Acids
- all are considered hydrophobic. However, tyrosine and tryptophan also
can form hydrogen bonds.
phenylalanine
tyrosine
tryptophan - containing an indole group
Sulfur-containing
- both are considered hydrophobic. Cysteine can form disulfide bonds with
another cysteine.
cysteine - containing a thiol group
methionine - containing a thioether group
Aliphatic hydroxyl-containing
serine
threonine - beta-branched, second chiral center
Basic
lysine - containing a primary amine
arginine - containing a guanidino group
histidine - containing an imidazole group
Acidic
aspartate
glutamate
Amide Derivatives
(of the acidic amino acids)
asparagine
glutamine
Peptide Bond Formation
attends the condensation of two amino acids with release of one molecule
of H2O. The peptide bond is a specialized form of amide bond. The reaction
is thermodynamically unfavorable. Both in nature and in the laboratory,
the reaction is driven by using activated derivatives of the amino acids
whose reactivity is much stronger.
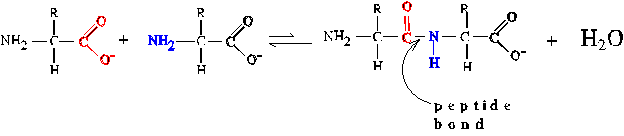
Post-translational Modification
After synthesis, proteins undergo important and often extensive covalent
modification, sometimes the removal of a polypeptide segment, sometimes
addition of a new chemical moiety (VVP, pp. 90, 256-258, 881-882). Often
such proteins will not be "active" until a modification has occurred (e.g.
phosphorylation). A modification can prolong the life of a protein (acetylation),
or shorten it (ubiquitination). Some modifications are reversible and others
are permanent. Many modifications are listed here, but they shall not be
covered in detail until later in the course. The structures of a few key
modifications are shown in VVP, p.90.
Proteolytic cleavage associated with protein sorting.
Proteins destined for transport into membrane-bound compartments such as
the mitochondrion and the endoplasmic reticulum typically are synthesized
with an amino-terminal targeting sequence that directs the protein
to the target organelle. The targeting sequences are usually removed by
proteolytic cleavage within the organelle.
In the Cytosol
-
acetylation of the amino terminus and the sidechain of lysine (VVP,
pp. 90, 761; Stryer, p. 977)
-
phosphorylation of serine, threonine, tyrosine in eukaryotes (examples:
VVP, pp. 440; Stryer, p. 590) and of histidine and aspartate in bacteria
(Stryer, p. 329)
-
myristoylation of the amino terminus (VVP, p. 245; Stryer, p. 933)
-
palmitoylation of cysteine (VVP, p. 245; Stryer, p. 933)
-
prenylation of the carboxy terminus (VVP, p. 245; Stryer, p. 933)
-
o-methylation of lysine and glutamate in bacteria and of
prenyl cysteine (VVP, p. 245; Stryer, p. 933)
-
ubiquitination of the amino terminus and lysine (VVP, p. 613; Stryer,
p. 942)
In the Secretory Pathway
-
disulfide bond formation (VVP, p. 83, 154-155; Stryer, p.25)
-
hydroxylation of proline and lysine (VVP, p. 90, 134-135; Stryer,
p. 31)
-
gamma-carboxylation of glutamate (VVP, p. 90; Stryer, p. 255)
-
glycosylation of asparagine, serine and threonine (VVP, p. 212-214;
Stryer, p. 920)
-
addition of a glycosyl phosphatidyl inositol (GPI) anchor
(VVP,
p. 245; Stryer, p. 935)
-
formation and crosslinking of allysine in collagen (VVP, p. 137)
Homework
Examine the four amino acids given below:
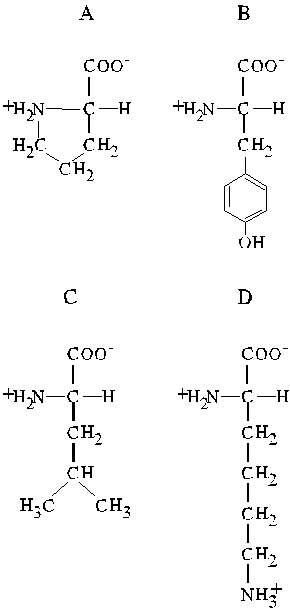
-
Name the four amino acids.
-
Name the other amino acids of the same class as D.
-
Draw the structure of cysteine at pH 1.
-
Name an example of a protein for each post-translational modification specified
above.
-
Compare and contrast acquisition of amino-terminal acetyl and formyl groups.
-
Name and draw the structure of an amino acid which is an example of the
following groups: (a) nonpolar aliphatic, (b) nonpolar aromatic, (c) basic,
(d) acidic, (e) sulfur-containing, (f) hydroxyl-containing.
-
List the twenty amino acids in order of hydrophobicity (use your own judgment).
-
Find a hydrophobicity ranking in the literature (textbook, Web site, etc.)
and compare it with your own ranking. Rationalize deviant amino acids.
-
How did earth-bound biological materials come to be dominated by L-amino
acids? Could it have gone the other way?
End of document