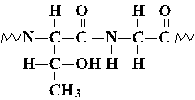Biochemistry 601
September 3, 1999 Protein Secondary and Tertiary Structure
Last modified 8/23/99
Dr. Landry
Rm. 6055
landry@mailhost.tcs.tulane.edu
Reading assignment:
Objectives
-
Recognize that the partial double-bond character of the peptide bond results
in its planarity.
-
See that steric interference
further limits polypeptide flexibility.
-
Recognize that formation of regular secondary structure is a direct consequence
of polypeptide chain collapse.
-
Realize that hydrogen bonding causes secondary structure formation to be
cooperative.
-
Identify the essential features of an alpha-helix.
-
Appreciate that beta-sheets are stabilized
by interactions between residues distant from each other in the amino acid
sequence.
-
Recognize that protein structures can be presented in different ways: alpha-carbon
trace, backbone, stick, ribbon, CPK (Cory-Pauling-Koltun).
-
Understand the hierarchy of protein structure: primary, secondary, tertiary,
quaternary.
-
Appreciate the diversity of tertiary structure types and know that there
are common classes, e.g., four-helix bundle, beta barrel, TIM barrel.
-
Recognize common themes in protein structure/function relationships,
e.g.,
DNA binding often is mediated by alpha-helices, and enzyme active sites
occur at the C-terminus of beta-strands in alpha/beta proteins (such as
triose phosphate isomerase).
-
Appreciate that the fibrous structure of collagen imposes unique demands
on the amino acid sequence.
Planarity of the Peptide Bond
The peptide bond is slightly shorter than a standard single bond, reflecting
the partial delocalization of pi electrons from the carbonyl group
into orbitals shared with the lone pair
electrons of the amide nitrogen.
This partial double-bond character inhibits rotation around the
peptide bond; thus, the four atoms bound to the carbonyl carbon and amide
nitrogen form a plane. A polypeptide chain may be considered as a series
of planes with two angles of rotation between each plane.
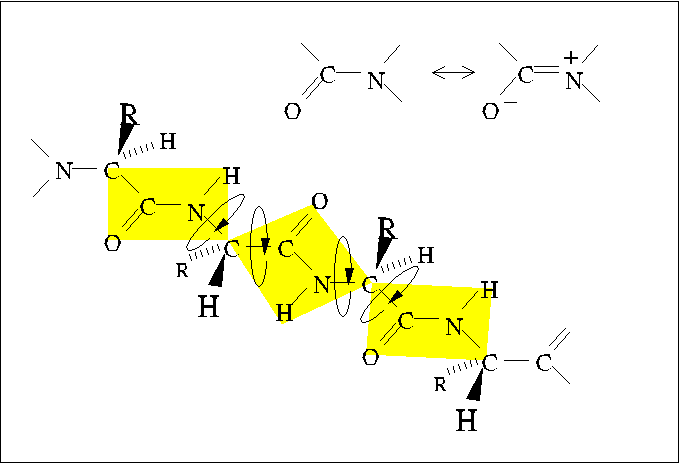
The backbone conformation of a polypeptide chain is effectively determined
by the values of three dihedral angles: Phi, Psi, and Omega.
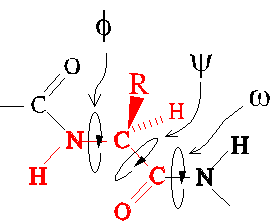
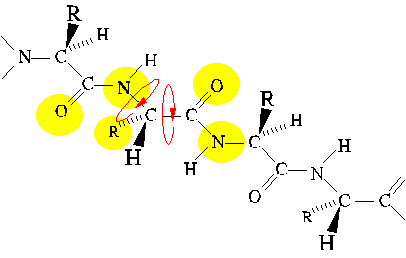
Steric interference
Allowed values of dihedral angles Phi, Psi and Omega are severly restricted
by steric interference.
The trans conformer of the peptide bond (Omega) is strongly favored
over the cis conformer, owing to steric interference between consecutive
sidechains.
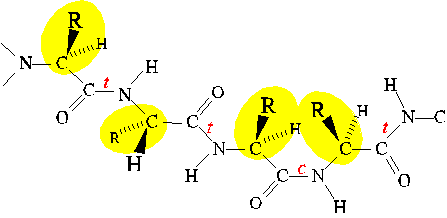
Similarly, Phi and Psi are restricted to values close to those of extended
and alpha-helical conformations. (Only the extended conformation is shown
here.)
Secondary Structure as a Consequence of Chain
Collapse
A simple theoretical model for polypeptides consists of hydrophilic and
hydrophobic beads on a string. This model is surprisingly successful in
its recapitulation of folding behavior. One outcome of the study of this
model was the realization that regular secondary structures, alpha helix
and beta sheet, are a direct consequence of chain collapse into
a compact space.
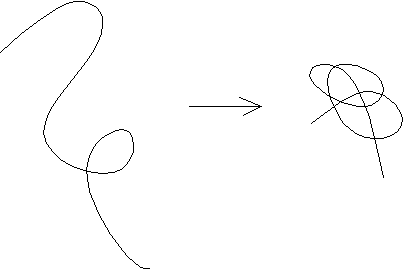
Thus, the amino acid sequence only influences the partitioning of segments
between secondary structure types. For example, alanine, leucine, lysine
and glutamate favor alpha helix; whereas, beta-branched amino acids threonine,
valine, and isoleucine favor beta sheet.
Regular Secondary Structure Elements
Alpha Helix
The alpha helix is a type of regular secondary structure in which
successive amino acids adopt the same Phi and Psi dihedral angles (peptide
bonds all trans). It is a coiled structure characterized by 3.6
residues per turn, and translating along its axis 1.5 angstrom per
amino acid. Thus the pitch is 3.6x1.5 or 5.4 angstrom. The screw
sense of alpha helices is always right-handed.
View the alpha-helical conformation with a kinemage.
In alpha helices, the CO of residue i is hydrogen-bonded to
the NH of residue i+4.
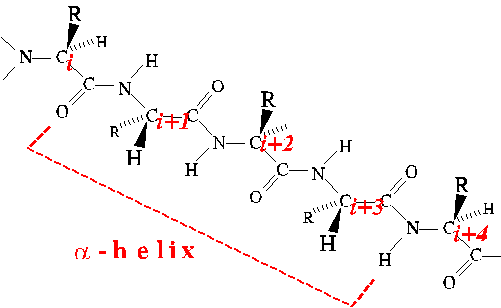
The Phi and Psi angles of four successive amino acids must adopt the
alpha-helical conformation in order to realize the stabilization of a single
H-bond. Thus there is a penalty for starting and ending alpha helices.
Once a helix is started, an H-bond is added for each additional residue
incorporated into the helix. As a result, helix formation is highly
cooperative.
The ends of alpha helices can be stabilized by end-capping. End
caps are sidechain-to-backbone H-bonds between the sidechain of a residue
just beyond the end of the helix and an otherwise unsatisfied backbone
H-bonding group of a residue in the helix.
View some "classic" end cap structures with a kinemage
by Seale et al.
The regular arrangement of peptide bonds results in an excess of partially
positively charged amide nitrogen atoms near the amino-terminus of a helix
and partially negatively charged oxygen atoms near the carboxy-terminus.
The aggregate charge separation is termed the helix dipole, and
it destabilizes the helix.
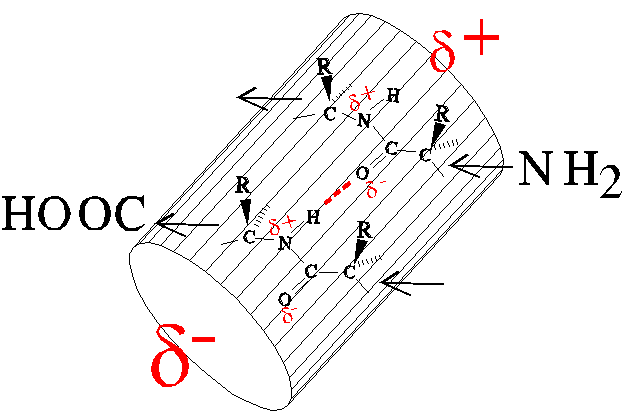
The compensating charge of an appropriate sidechain can stabilize the
helix, e.g., glutamate at the amino-terminus or lysine at the carboxy-terminus.
Some proteins use the helix dipole to stabilize binding of charged ligands.
See the kinemage about nucleotide-binding enzymes.
Beta Sheet
Beta sheet also is regular secondary structure formed by successively repeated
Phi and Psi angles. Importantly, however, the H-bonding pattern is not
regularly spaced with respect to the amino acid sequence. H-bonds span
between amino acids on separate beta strands, which may be quite
distant from each other in the sequence. For the structure shown in this
kinemage
on beta structure, H-bonds extend between following residues: 4-13
(NH-CO), 4-13 (CO-NH), 6-11 (NH-CO), and 6-10 (CO-NH). This is an example
of
antiparallel beta sheet. The two beta strands are separated by
a reverse turn, a type of non-regular secondary structure. Refer
again to the kinemage about nucleotide-binding enzymes
to see an example of parallel beta sheet, whose strands must be
separated by some length of intervening structure such as alpha helix.
Sample Questions
1. How many different dipeptides can be made from the 20 L amino acids?
What are the minimum and the maximum number of pK values for any dipeptide?
2. For the pentapeptide Glu-Met-Arg-Thr-Gly,
(a) name the carboxyl-terminal residue:
(b) give the number of charged groups at pH 7:
(c) give the net charge at pH 1:
(d) draw the peptide bond between the Thr and Gly residues, including
both side chains.
3. If a polypeptide has 400 amino acid residues, what is the approximate
mass?
(a) 11,000 daltons
(b) 22,000 daltons
(c) 44,000 daltons
(d) 88,000 daltons
4. Which amino acid can stabilize protein structures by forming covalent
cross-links between polypeptide chains?
(a) Met
(b) Ser
(c) Gln
(d) Gly
(e) Cys
5. Which of the following statements about the peptide bond are true?
(a) The peptide bond is planar because of the partial double-bond character
of the bond between the carboxyl carbon and the nitrogen.
(b) There is relative freedom of rotation of the bond between the carboxyl
carbon and the nitrogen.
(c) The hydrogen that is bonded to the nitrogen atom is trans to the
oxygen of the carboxyl.
(d) There is no freedom of rotation around the bond between the alpha
carbon and the carboxyl carbon.
6. Which of the following properties are shared by alpha-helical and
beta pleated sheet structures in proteins?
(a) Rod shape
(b) Hydrogen bonds between main-chain CO and NH groups
(c) Axial distance between adjacent amino acids of 3.5 angstroms
(d) Variable numbers of participating amino acid residues
7. Explain why Gly occurs every third residue in the sequence of collagen.
Answers
-
400; two pK's min, four max.
-
(a) gly; (b) 4; (c) +2; (d)
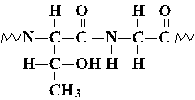
-
c
-
e
-
a, c
-
b, d
-
Since there are three residues per turn of helix, every third residue pints
inward. The interior residues are Gly because Gly is the only residue small
enough to fit inside the superhelical cable.
Examples of Tertiary and Quaternary Protein Structure
-
File #4 - TERTIARY STRUCTURE
-
THE FOUR HELIX BUNDLE (HEMERYTHRIN)
-
File #6 - TERTIARY STRUCTURE
-
THE BETA BARREL (BACTERIOCHLOROPHYLL PROTEIN)
-
File #7 - TERTIARY STRUCTURE
-
A MEMBRANE SPANNING BETA-BARREL (PORIN)
-
File #8 - MIXED TERTIARY STRUCTURE MOTIFS
-
THE ZINC FINGER
-
File #9 - MIXED TERTIARY STRUCTURE MOTIFS
-
THE ALPHA/BETA BARREL (A COMMON MOTIF)
-
Ribbon
diagram of chicken TIM showing the ligand bound at the C-terminal end of
the beta strands
-
File #10 - MIXED TERTIARY STRUCTURE MOTIF
-
THE ALPHA/BETA FOLD OF IMPORTANT SIGNALING PROTEINS IN THE HUMAN BODY
(Ras PROTEIN)
-
File #11 - QUATERNARY STRUCTURE
-
THE COILED-COIL (LEUCINE ZIPPER)
-
File #12 - QUATERNARY STRUCTURE
-
ASSOCIATION OF TWO IDENTICAL CHAINS (HIV PROTEASE)
-
File #13 - QUATERNARY STRUCTURE
-
ASSOCIATION OF INEQUIVALENT SUBUNITS AND EVOLUTIONARY CONSERVATION (HEMOGLOBIN)
-
File #14 - QUATERNARY STRUCTURE
-
ASSOCIATION OF THREE INEQUIVALENT SUBUNITS (PHOTOREACTION CENTER PROTEIN)
-
File #16 - THE DISULFIDE BRIDGE
-
STRUCTURAL STABILIZATION OF PROTEINS
-
File #17 - PROTEIN-DNA INTERACTIONS
-
LAMBDA REPRESSOR
-
File #18 - PROTEIN-DNA INTERACTIONS
-
A ZINC FINGER PROTEIN
End of Document