| Additional
Plasmodium Species Infecting Humans? |
- parasites
with identical morphologically as P. vivax (1)
- CSP sequence
= P. simiovale
- variant
is found globally
- morphological
variants of P. malariae (2)
- parasites
with distinct morphologies identified in SE Asia
- molecular
sequences similar to P. malariae/P. brasilianum
- possibly
related to P. vivax var. minuta (3) or P. tenue
(4)
- naturally
acquire P. knowlesi infections (5)
- P.
ovale
clades as distinct species (6)
- Qari
et al (1993) J. Inf. Dis. 168:1485
- Kawamoto
et al (2002) J. Parasitol. 88:350
- Craig
(1914) J. Parasitol. 1:85
- Stephens
(1914) Proc. R. Soc. Lond. (Series B) 87:375
- Cox-Singh
et al (2008) Clinical Infectious Disease 46:165
- Sutherland
et al (2010) J. Inf. Dis. 201:1544
|
Four distinct
Plasmodium species infect humans: P. falciparum, P. vivax,
P. malariae, and P. ovale. However, molecular
methods have revealed the possible existence of other species or morphological
variants (see box). For example, sequencing of the gene for the circumsporozoite
surface protein (CSP) revealed that some individuals diagnosed with P. vivax
infections were actually infected with a distinct species more closely related
to P. simiovale, a simian malaria parasite which is morphologically
identical to P. vivax. In addition, molecular analysis indicates that
P. ovale consists of two clades that are as divergent as distinct species and Sutherland et al (2010) have proposed to designate them as sub-species: P.o. curtisi and P.o. wallikeri.
Similarly,
molecular analyses indicate that some morphological variants of P. malariae
are distinct parasites related to P. malariae and P. brasilianum.
P. brasilianum is a simian parasite of the South and Central America
that is often speculated to have originated from humans as a result of colonization
of the New World.
In addition,
humans naturally infected with the simian parasite P. knowlesi have
been identified in Malaysia. Nearly all of the cases identified by microscopy
as P. malariae were determined to be P. knowlesi by PCR. Four
fatalities associated with P. knowlesi infection were also reported
from Malysia. Humans infected with P. knowlesi may not be such an rare
occurance and may be widespread in Malaysia and perhaps other parts of southeast
Asia (Cox-Singh
and Singh, 2008).
The four major
human Plasmodium species are found in tropical and subtropical regions
throughout the world and exhibit overlapping geographical
distributions. Differences between the species include:
- blood-stage
morphology
- minor life cycle variations
- P. vivax and
P. ovale exhibit the hypnozoite stage and can cause true relapses
(see discussion of hypnozoites and relapses
in main document)
- trophozoite- and schizont-infected
erythrocytes of P. falciparum sequester in the microvasuculature
and are not found in the peripheral circulation (see discussion of cytoadherence
in main document)
- host erythrocyte preference
- P. vivax and
P. ovale prefer reticulocytes (immature erythrocytes)
- P. malariae
prefers senescent erythrocytes
- P. falciparum
exhibits no preference
- disease
and clinical manifestation
| Species |
Older
Designation |
Newer
Designation |
|
| falciparum |
malignant
tertian |
falciparum
malaria |
| vivax |
benign
tertian |
vivax
malaria |
| ovale |
ovale
tertian |
ovale
malaria |
| malariae |
quartan |
quartan |
|
The older designations (Table)
for the various types of malaria reflect the differences in the diseases caused
by the different Plasmodium species. P. falciparum causes the
most severe disease, hence the malignant designation. This increase morbidity
and mortalilty correlates with the higher parasitemia associated with P.
falciparum infections and the complications arising from sequestration (see
Pathogenesis and Cerebral Malaria). Factors
contributing to the higher parasitemias include: large number of merozoites
per schizont, lack of host erythrocyte preference, and the immune evasion (ie,
spleen avoidance) provided by sequestration. Tertian and quartan refer to the
differences in the periodicity of paroxysms (see discussion of paroxysms).
Tertian patterns exhibit 48 hour periodicities and quartan refers to a 72 hour
periodicity. (Note that in Roman counting the first attack is on day one followed
of a symptom-free day and then the next attack on day three.) The species also
exhibit other differences in disease severity and duration (see Table
in main document).
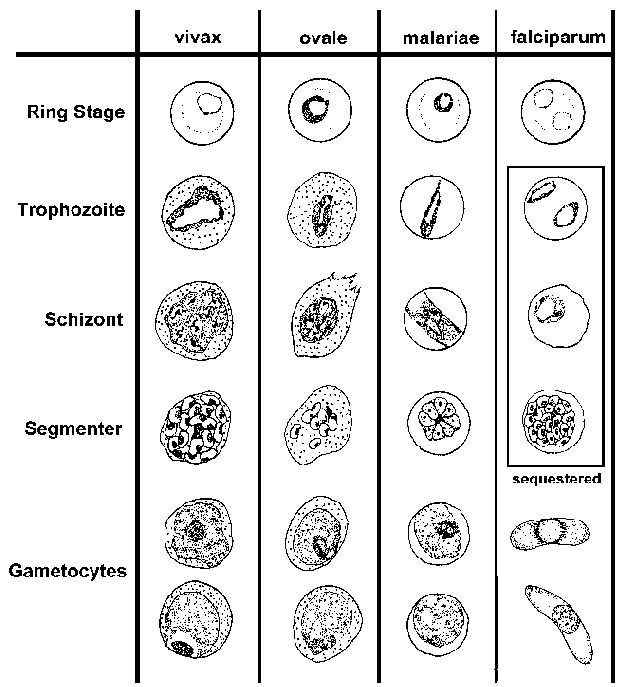
The blood-stage parasites
of human Plasmodium species exhibit differences in their morphology and
modify the host erythrocyte differently (see Table and Figure). These differences
can be used to distinguish the four species. (See life
cycle for description of blood-stage forms.)
| Key
Morphological Differences Between Human Plasmodium Species in Blood
Smears |
| falciparum |
vivax |
ovale |
malariae |
- numerous rings
- smaller rings
- no trophozoites or schizonts
- cresent-shaped gametocytes
|
- enlarged erythrocyte
- Schüffner's dots
- 'ameboid' trophozoite
|
- similar to P. vivax
- compact trophozoite
- fewer merozoites in schizont
- elongated erythrocyte
|
- compact parasite
- merozoites in rosette
|
P. falciparum blood smears are characterized by the presence of young trophozoites (ie, rings) in the absence of mature trophozoites and schizonts. The ring stages of P. falciparum tend to be slightly smaller than the other species and are generally more numerous. Multiply infected erythrocytes and appliqué forms are seen more often in P. falciparum than in the other species. The crescent-shaped gametocytes of P. falciparum are very distinctive, but tend to only appear late in the infection.
The most distinctive features of P. vivax are the enlarged infected erythrocytes and the appearance of granules, called 'Schüffner's dots', over the erythrocyte cytoplasm. These granules are manifestation of caveola-vesicle complexes that form on the erythrocyte membrane. The growing trophozoite of P. vivax often has an ameboid apearance and the schizonts can have more than 20 merozoites.
P. ovale also exhibits Schüffner's dots and an enlarged erythrocyte, making it difficult to distinguish from P. vivax. In general, P. ovale is a more compact parasite than P. vivax. This compactness is most evident in the growing trophozite stage and fewer merozoites are found per schizont. P. ovale also has more of a tendency to form elongated host erythrocytes.
P. malariae is characterized by a compact parasite (all stages) and does not alter the host erythrocyte or cause enlargment. Elongated trophozoites stretching across the erythrocyte, called band forms, are sometimes observed. Schizonts will typically have 8-10 merozoites that are often arranged in a rosette pattern with a clump of pigment in the center.