| EENS 2110 |
Mineralogy |
| Tulane University |
Prof. Stephen A. Nelson |
|
Silicate Structures,
Structural Formula, |
|
|
|
|
Silicate Structures and Structural Formula |
| As we discussed in a previous lecture, the relative abundance of elements in the Earth's crust determines what minerals will form and what minerals will be common. Because Oxygen and Silicon are the most abundant elements, the silicate minerals are the most common. Thus, we will spend some time here discussing the structure, chemistry, and occurrence of silicate minerals. Our systematic discussion of the common rock forming minerals will follow in the lectures throughout the remainder of the course. |
|
| In order to discuss the silicates and their structures it is first necessary to remember that the way atoms are packed together or coordinated by larger anions, like oxygen depends on the radius ratio of the cation to the anion, Rx/Rz. |
|
| Since oxygen is the most abundant element in the crust, oxygen will be the major anion that coordinates the other other cations. Thus, for the major ions that occur in the crust, we can make the following table showing the coordination and coordination polyhedra that are expected for each of the common cations. |
Ion C.N.
(with Oxygen)Coord. Polyhedron Ionic Radius, Å K+ 8 - 12 cubic to closest 1.51 (8) - 1.64 (12) Na+ 8 - 6 cubic to octahedral 1.18 (8) - 1.02 (6) Ca+2 8 - 6 1.12 (8) - 1.00 (6) Mn+2 6 Octahedral 0.83 Fe+2 6 0.78 Mg+2 6 0.72 Fe+3 6 0.65 Ti+4 6 0.61 Al+3 6 0.54 Al+3 4 Tetrahedral 0.39 Si+4 4 0.26 C+4 3 Triangular 0.08
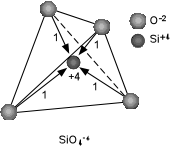
| The radius ratio of Si+4 to O-2 requires that Si+4 be coordinated by 4 O-2 ions in tetrahedral coordination. |
| In order to neutralize the +4 charge on the Si cation, one negative
charge from each of the Oxygen ions will reach the Si cation. Thus, each
Oxygen will be left with a net charge of -1, resulting in a SiO4-4
tetrahedral group that can be bonded to other cations. It is this
SiO4-4 tetrahedron that forms the basis of the
silicate minerals.
|
 |
| Since Si+4 is a highly charged cation, Pauling's rules state that it should be separated a far as possible from other Si+4 ions. Thus, when these SiO4-4 tetrahedrons are linked together, only corner oxygens will be shared with other SiO4-4 groups. Several possibilities exist and give rise to the different silicate groups. |
Nesosilicates (Island Silicates) If the corner oxygens are not shared with other SiO4-4 tetrahedrons, each tetrahedron will be isolated. Thus, this group is often referred to as the island silicate group. The basic structural unit is then SiO4-4. In this group the oxygens are shared with octahedral groups that contain other cations like Mg+2, Fe+2, or Ca+2. Olivine is a good example: (Mg,Fe)2SiO4.
|
|
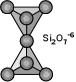
Sorosilicates (Double Island Silicates) If one of the corner oxygens is shared with another tetrahedron, this gives rise to the sorosilicate group. It is often referred to as the double island group because there are two linked tetrahedrons isolated from all other tetrahedrons. In this case, the basic structural unit is Si2O7-6. A good example of a sorosilicate is the mineral hemimorphite - Zn4Si2O7(OH).H2O. Some sorosilicates are a combination of single and double islands, like in epidote - Ca2(Fe+3,Al)Al2(SiO4)(Si2O7)(OH). |
 |
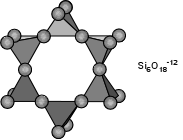
Cyclosilicates (Ring Silicates) If two of the oxygens are shared and the structure is arranged in a ring, such as that shown here, we get the basic structural unit of the cyclosilcates or ring silicates. Shown here is a six membered ring forming the structural group Si6O18-12. Three membered rings, Si3O9-6, four membered rings, Si4O12-8, and five membered rings Si5O15-10 are also possible. A good example of a cyclosilicate is the mineral Beryl - Be3Al2Si6O18. |
 |
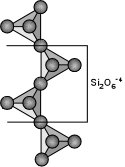
Inosilicates (Single Chain Silicates) If two of the oxygens are shared in a way to make long single chains of linked SiO4 tetrahedra, we get the single chain silicates or inosilicates. In this case the basic structural unit is Si2O6-4 or SiO3-2. This group is the basis for the pyroxene group of minerals, like the orthopyroxenes (Mg,Fe)SiO3 or the clinopyroxenes Ca(Mg,Fe)Si2O6. |
 |
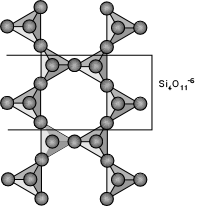
Inosilicates (Double Chain Silicates) If two chains are linked together so that each tetrahedral group shares 3 of its oxygens, we can from double chains, with the basic structural group being Si4O11-6. The amphibole group of minerals are double chain silicates, for example the tremolite - ferroactinolite series - Ca2(Mg,Fe)5Si8O22(OH)2.
|
 |
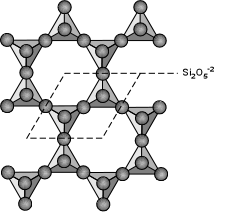
Phyllosilicates (Sheet Silicates) If 3 of the oxygens from each tetrahedral group are shared such that an infinite sheet of SiO4 tetrahedra are shared we get the basis for the phyllosilicates or sheet silicates. In this case the basic structural group is Si2O5-2. The micas, clay minerals, chlorite, talc, and serpentine minerals are all based on this structure. A good example is biotite - K(Mg,Fe)3(AlSi3)O10(OH)2. Note that in this structure, Al is substituting for Si in one of the tetrahedral groups. |
 |
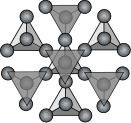
Tectosilicates (Framework Silicates) If all of the corner oxygens are shared with another SiO4 tetrahedron, then a framework structure develops. The basic structural group then becomes SiO2. The minerals quartz, cristobalite, and tridymite all are based on this structure. If some of the Si+4 ions are replaced by Al+3 then this produces a charge imbalance and allows for other ions to be found coordinated in different arrangements within the framework structure. Thus, the feldspar and feldspathoid minerals are also based on the tectosilicate framework.
|
 |
| General Formula for Silicates
Based on these basic structural units, we can construct a general structural chemical formula for the silicates. But one substitution in particular tends to mess things up a bit. This is Al+3, the third most abundant element in the Earth's crust. Al+3 has an ionic radius that varies between 0.54 and 0.39 depending on the coordination number. Thus, it could either fit in 6-fold coordination with oxygen or 4-fold coordination with oxygen. Because Al+3 will go into 4-fold coordination with oxygen, it sometimes substitutes for Si+4. If such a substitution takes place, it creates a charge imbalance that must be made up elsewhere in the silicate structure.
The other common elements in the Earth's crust that enter the silicates do so in other types of coordination. Ions like Al+3, Mg+2, Fe+2, Fe+3, Mn+2, and Ti+4 enter into 6-fold or octahedral sites. Larger ions like Ca+2, and Na+1, are found in octahedral coordination or 8-fold, cubic coordination sites. Very large cations like K+1, Ba+2, and sometimes Na+1 are coordinated by 12 oxygens in 12-fold coordination sites. We can thus write a general structural formula for the silicates as follows: XmYn(ZpOq)Wr where X represents an 8 to 12 fold coordination site for large cations like K+, Rb+, Ba+2, Na+, and Ca+2.
Y represents a 6-fold (octahedral) site for intermediate sized
cations like Al+3, Mg+2, Fe+2, Fe+3,
Mn+2, and Ti+4. |
|
the ratio p:q depends on the degree of polymerization of the silica (or alumina) tetrahedrons, or the silicate structural type as discussed above. O is oxygen, and W is a hyrdoxyl (OH-1) site into which can substitute large anions like F-1 or Cl-1. The subscripts m, n, and r depend on the ratio of p to q and are chosen to maintain charge balance.
This is summarized in the table shown here. In this table note that there is very little substitution that takes place between ions that enter the X, Y, and Z sites. The exceptions are mainly substitution of Al+3 for Si+4, which is noted in the Table, and whether the X site is large enough to accept the largest cations like K+1, Ba+2, or Rb+1. |
|
|
Nesosilicates (Island Silicates)
We now turn our discussion to a systematic look at the most common rock forming minerals, starting with the common nesosilicates. Among these are the olivines, garnets, Al2SiO5 minerals, staurolite, and sphene (the latter two will be discussed in the last lecture on accessory minerals). |
| As discussed above, the nesosilicates or island silicates are based on the isolated SiO4-4 tetrahedral groups. In the olivines, the remaining corner oxygens form octahedral groups that coordinate Mg+2 and Fe+2 ions. |
|
Olivines The olivines consist of a complete solid solution between Mg2SiO4 (forsterite, Fo) and Fe2SiO4 (fayalite, Fa). There is limited substitution of the following end members:
Ca2SiO4 - larnite Mn2SiO4 - tephroite CaMgSiO4 - monticellite (which is commonly found in metamorphosed dolomites)
|
| The phase diagram for the common end members of the olivine solid solution series shows that pure forsterite melts at 1890oC and pure fayalite melts at 1205oC. Thus, the olivines are sometimes seen be be zoned from Mg-rich cores to more Fe-rich rims, although such zoning is usually limited to 5 to 10% difference between the cores and the rims. | 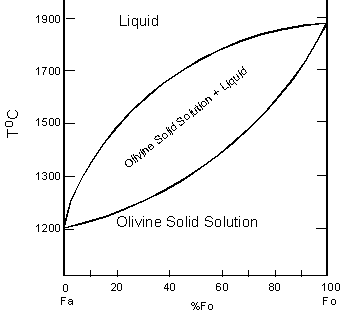 |
|
| Garnets
Garnets are isometric minerals and thus isotropic in thin section, although sometimes they are seen to be weakly birefringent (slightly anisotropic). They are also nesosilicates, and therefore based on the SiO4 structural unit. The general formula for garnets is:
Garnets with no Ca in the A site and Al in the B site are called the pyralspite series. These consist of the end members:
Garnets with Ca in the A site are called the ugrandite series and
consist of the end members:
Limited solid solution exists between end members of each series.
|
Al2SiO5 Minerals The Al2SiO5 minerals are common in aluminous metamorphic rocks (meta-shales and meta-mudstones) and sometimes found in aluminous igneous rocks. |
| In metamorphic rocks the Al2SiO5 polymorphs provide rather general estimates of the pressure and temperature of metamorphism, with Kyanite indicating relatively high pressure, andalusite indicating low temperature and pressure, and sillimanite indicating high temperature. Better estimates of pressure and temperature are provided if two of the minerals are present in the same rock. | 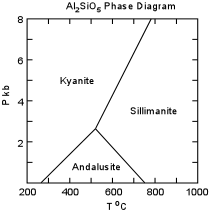 |
|
 |
|
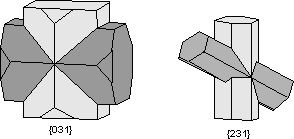
Staurolite (Mg,Fe)2Al9Si4O22(OH)2 Staurolite is a common mineral in medium grade metamorphic rocks, usually metamorphosed shales. In hand specimen and in thin section it characteristically is seen to show staurolite twinning, either the right-angle cross, twinned on {031} or the oblique cross, twinned on {231} |
 |
| It is monoclinic, but its optical properties are those of an orthorhombic mineral. It has moderate {010} cleavage, which if present, will cause parallel extinction. It's most distinguishing property is its pleochroism, with α = colorless, β = pale yellow, and γ = golden yellow. Less distinctive are its positive optic sign and 2V = 82 - 90o. In many rocks Staurolite shows twinning, and commonly forms euhedral crystals with well developed {100} and {010} crystal faces. In thin section Staurolite is commonly seen to contain tiny inclusions of other minerals, usually quartz. There are very few minerals which can be confused with Staurolite. |
Zircon ZrSiO4 Zircon is a common accessory mineral in nearly all kinds of rocks, particularly the more siliceous igneous rocks, like granites, granodiorites, and syenites. Still, it is not often found in thin section because it is so hard that it gets plucked out during the grinding of the section. Zircon usually contains high amounts of radioactive elements like U and Th. Thus, when it is found as inclusions in minerals like biotite, it produces pleochroic haloes in the biotite as seen in thin section. Because it contains high concentrations of U and Th, it is very useful in obtaining U-Pb and Th-Pb radiometric dates on old rocks. It is very resistant to weathering and may also survives during metamorphism, allowing for dates to be obtained on the original rock prior to metamorphism (often called the protolith). In hand specimen Zircon usually occurs as tiny reddish colored crystals. In thin section, it shows extremely high relief, with ω = 1.923 to 1.960 and ε = 1.968 to 2.015. and is uniaxial positive. Zircon has high birefringence, with interference colors in the higher orders (lots of reds, pinks and light greens). It is commonly colorless to pale brown or pinkish brown in polarized light without the analyzer. Generally it occurs as small crystals with relief higher than almost anything else in the thin section. This latter property should tip you off to its presence. Sphene (Titanite) CaTiSiO4(OH) Sphene is another common accessory mineral in plutonic igneous rocks like granites, granodiorites, and syenites. It is also found as larger crystals in metamorphic gneisses and chlorite bearing schists. In hand specimen as an accessory mineral, it is usually seen as small wedge-shaped crystals with a resinous to adamantine luster and brown to yellow brown color. In thin section, Sphene, has a relief similar to that of zircon, and is usually found in small crystals with an elongated diamond shape. It is generally brownish in color, shows a well developed {110} cleavage, and high order interference colors. |
|
Sorosilicates Sorosilicates are the double island silicates. Only one important mineral group, the epidote group, has this structure. Epidote, Clinozoisite, Zoisite The important minerals in the epidote group are epidote, clinozoisite, and zoisite. Since the sorosilicates are based on the Si2O7 -6 group, the structural formula can be written as:
Thus, the epidote group contains both the double tetrahedra and the single tetrahedron, separated by groups of AlO6 octahedra and Ca in nine to 10 fold coordination with Oxygen or OH. The formula can be rewritten as:
Epidote is the Fe-rich variety and has the above general formula. Clinozoisite is the Fe-free variety with the chemical formula:
Both clinozoisite and epidote are monoclinic (2/m). Zoisite has the same chemical formula as clinozoisite, but is orthorhombic. Epidote is usually pistachio green in color with perfect {001} cleavage and imperfect {100} cleavage. It is optically negative with a 2V of 64 - 90o. It usually shows pleochroism with α - colorless to pale yellow, β - greenish yellow, and γ - yellowish green, and shows high relief relative to feldspars and quartz. It's birefringence is high enough to show 3rd order interference colors. It usually shows an anomalous blue extinction. Clinozoisite shows similar relief and cleavage to epidote, but it is optically negative with a 2V of 14 to 90o, shows no pleochroism, and lower birefringence (1st to 2nd order interference colors). Zoisite is similar to clinozoisite, except it will show parallel extinction relative to faces parallel to the crystallographic axes. Epidote is a common mineral in low grade metamorphic rocks, particularly metamorphosed volcanic rocks and Fe-Al rich meta shales. Both Clinozoisite and epidote occur as alteration products of plagioclase and as veins in granitic rocks.
|
|
Cyclosilicates |
|
| The cyclosilicates are based on rings of SiO4 tetrahedra, with a Si:O ratio of 1:3 The most common minerals based on this structure are Beryl, Cordierite, and Tourmaline. |
 |
|
Beryl Be3Al2Si6O18 is hexagonal
(6/m2/m2/m) with a strong prismatic habit with the form {10 In thin section, Beryl shows higher relief than quartz, and is distinguished from quartz by its negative optic sign and length-fast character. The only other mineral that it can be confused with is apatite, but apatite shows even higher relief than Beryl. |
|
|
Cordierite is (Mg,Fe)2Al4Si5O18.nH2O. It is orthorhombic (2/m2/m2/m), but shows a pseudohexagonal character due to its common cyclical twinning on {110}. In thin section it may show a twinning that looks like albite twinning, which makes it hard to distinguish from plagioclase. But, cordierite is usually dusted with tiny opaque inclusions. In thick sections it shows α pale -yellow, violet, pale blue pleochroism. It can be distinguished from quartz by its biaxial character. Cordierite is a common constituent of aluminous metamorphic rocks. It is common in contact metamorphic rocks where it is commonly associated with sillimanite or andalusite, feldspars and micas. Tourmaline Tourmaline
- Na(Mg,Fe,Mn,Li,Al)3Al6Si6O18(BO3)3(OH)4
is hexagonal (3m) and is commonly found as
well-formed prismatic crystals, with a rounded triangular cross section
perpendicular to the c crystallographic axis. Tourmaline is a common mineral in pegmatites (SiO2 - rich igneous rocks with large grain size), where it is associated with quartz and alkali feldspar. It is also found in metasomatized rocks of all types, where it is precipitated from a Boron and Silica - rich fluid phase. It's most distinguishing properties are its uniaxial negative optical character and its pleochroism with
ω = dark green or dark blue and
ε = yellow or violet. Tourmaline
usually forms in euhedral crystals with well developed prism faces and extinction parallel to the prism faces. |
Examples of questions on this material that could be asked on an exam (note that properties that distinguish different minerals will be included in the laboratory exam)
|