
Ph.D. (LSU, 1988)
1430 Tulane Ave., Box SL-43
New Orleans LA 70112
 |
Ph.D. (LSU, 1988) |
| Phone: (504) 988-3990 | |
| FAX: (504) 988-2739 | |
| Address: 1430 Tulane Ave., Box SL-43 New Orleans LA 70112 |
|
| Email: landry@tulane.edu |
Our work employs biochemical and biophysical techniques to investigate
structure/function
relationships in protein-protein interactions, especially those
involved
in cellular stress and immune responses.
Structural Instability and T-cell Epitope Immunodominance.
Humoral and cellular immunity to HIV and other pathogens depend on
antigen-specific
CD4+ T cell responses directed against peptides presented in MHC class
II antigen presenting proteins. Generally, CD4+ T-cell responses are
focused
on only a few immunodominant regions of naturally processed antigens.
We
have found that immunodominant regions occur in the sequences flanking
locally unstable segments of an antigen's three-dimensional
structure. We are now modifying CD4+ helper T-cell immune
responses to
recombinant
antigens by engineering their processing and presentation, and studying how the specificity of the CD4+ T-cell
response influences the effector phases of the immune response.
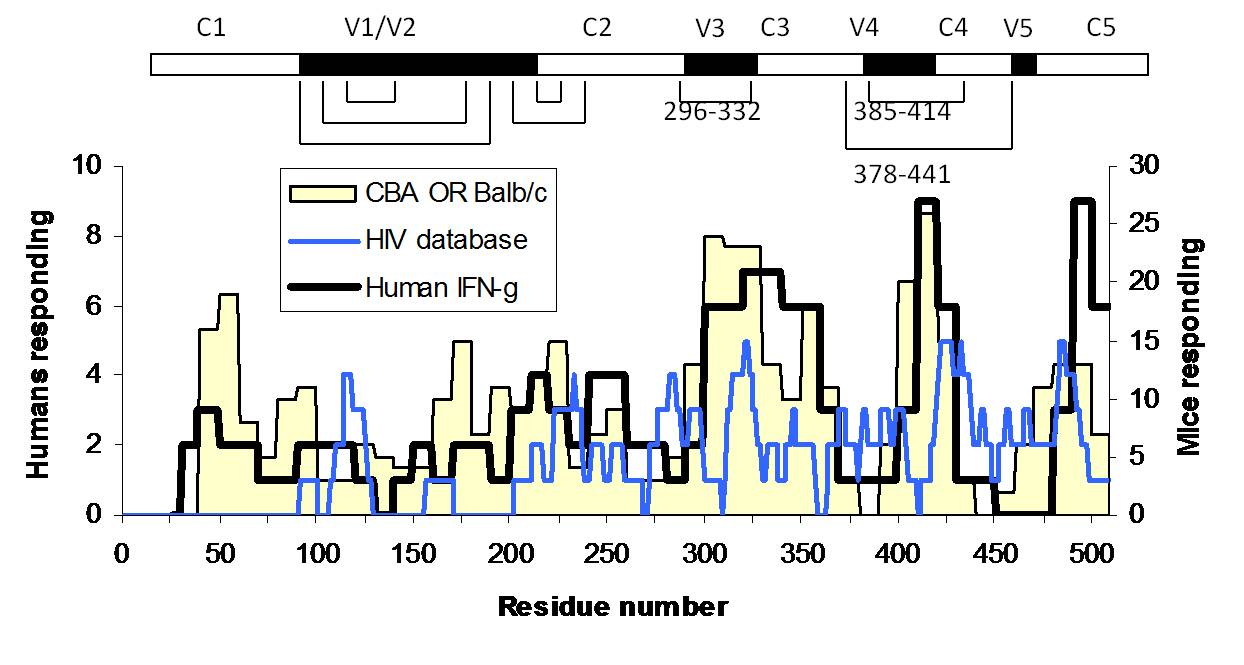 In our work on T-cell epitope immunodominance, we investigate how
the
immune system zooms in on key features of foreign and self proteins, as
it fights off invaders but avoids causing autoimmune disease. The
immune system recognizes bacteria, viruses, and toxins through
surveillance
by antibodies and T-cell receptors. Antibodies act alone to
recognize
intact foreign antigen molecules; whereas, T-cell receptors recognize
pieces
(epitopes) of antigen molecules that have been broken down and
presented
on the surface of specialized antigen-presenting cells. This
dual-aspect
recognition nearly ensures that the immune system will not attack our
own
molecules. Our work has shown that the process of antigen
breakdown
tends to focus the immune system on certain pieces of proteins.
We
suspect that bacteria and viruses have evolved to misdirect the immune
system toward highly variable or otherwise less effective protein
segments.
We are attempting to overcome this misdirection by engineering subunit
vaccines so that the immune system focuses on the most important
antigen
segments. Understanding how the immune systems distinguishes self
from non-self will allow us to develop strategies to treat and prevent
asthma and other forms of allergy.
In our work on T-cell epitope immunodominance, we investigate how
the
immune system zooms in on key features of foreign and self proteins, as
it fights off invaders but avoids causing autoimmune disease. The
immune system recognizes bacteria, viruses, and toxins through
surveillance
by antibodies and T-cell receptors. Antibodies act alone to
recognize
intact foreign antigen molecules; whereas, T-cell receptors recognize
pieces
(epitopes) of antigen molecules that have been broken down and
presented
on the surface of specialized antigen-presenting cells. This
dual-aspect
recognition nearly ensures that the immune system will not attack our
own
molecules. Our work has shown that the process of antigen
breakdown
tends to focus the immune system on certain pieces of proteins.
We
suspect that bacteria and viruses have evolved to misdirect the immune
system toward highly variable or otherwise less effective protein
segments.
We are attempting to overcome this misdirection by engineering subunit
vaccines so that the immune system focuses on the most important
antigen
segments. Understanding how the immune systems distinguishes self
from non-self will allow us to develop strategies to treat and prevent
asthma and other forms of allergy.
Former
Graduate Students
Current
List of publications.
| Tulane Biochemistry Home |