| Nature 407, 457 - 458 (2000) © Macmillan Publishers Ltd. |
LINDA PARTRIDGE1 AND NICHOLAS H. BARTON2
1
Linda Partridge is in the Department of Biology, University College London,
Wolfson House, 4 Stephenson Way, London NW1 2HE, UK.
e-mail: ucbhlop@ucl.ac.uk
2
Nicholas H. Barton is in the Institute for Cell, Animal and Population
Biology, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK.
e-mail: n.barton@ed.ac.uk
In yeast, a modified protein known as a prion generates variation in growth rate across diverse environments. Is this an example of an agent that has evolved in order to promote its possessor's adaptability?
The mechanism of natural selection is well understood for genes that affect the survival and reproductive success of their bearers. Selection can also act on genes that likewise affect their bearer's relatives, or (less effectively) on the group of which the bearer is a member. But can selection favour genes or other factors that help their bearer adapt to changing circumstances? That is, can evolvability itself evolve? Such genes would be subject to a different, much weaker, kind of selection.
There is nonetheless the possibility that modifying factors may increase the rate of mutation or of genetic recombination (the shuffling of genes that occurs during sexual reproduction). Such factors can increase in frequency if they can hitch-hike by association with the new, advantageous mutations1 or gene combinations2 that they create. There are two problems, however. First, most new mutations and gene combinations lower reproductive success rather than increase it. Second, if reproduction is sexual, the modifier will tend to be parted by recombination from the novelty it has caused.
So much for the problems. What evidence do we have that such modifying factors exist? One such mechanism for generating variability has been brought to light by True and Lindquist, as they describe on page 477 of this issue3. They have been working with a yeast prion that they suggest is maintained by natural selection because it aids evolution.
The prion, called [PSI+], is a self-propagating and inherited modification of a protein, Sup35, that is involved in terminating protein translation — the process by which proteins are manufactured by translation of the messenger RNA codons into an amino-acid sequence (Fig. 1a). The prion occurs naturally, and yeast cells switch spontaneously back and forth between the prion and wild-type (normal) conformation of the protein. The prion form of Sup35 makes inactive 'termination complexes' that can capture newly made, wild-type Sup35 protein and convert it into the prion form. The prion causes 'read-through' of experimentally introduced stop codons, and presumably also of some naturally occurring stop codons. Because these codons signal a halt to translation, read-through of them results in proteins with an extra segment ( Fig. 1b). In principle, this provides the potential for evolutionary change.
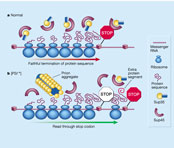 |
Figure 1 Protein production and the effects of the Sup35 protein and its modified form, the [PSI+] prion, in yeast. Full legend |
One end of Sup35 is essential for its role in terminating translation and for survival of the cell. The other end and middle (N and M) regions are required for the protein to assume the prion conformation. If these regions are deleted in cells with the prion, the prion conformation is lost. But there are no other gross effects, such as death of the cell, raising the issue of how these regions are maintained by selection.
True and Lindquist3 tested the idea that the prion introduces novel characteristics into yeast cells. They measured the growth rate of yeast strains in different environments, such as on different carbon and nitrogen sources. They used a set of pairs of strains that differed from one another only in the presence of wild-type or prion protein conformation, while each pair of strains differed in genetic background and in the extent of read-through of stop codons. In nearly half of 150 tests, the prion altered the growth rate, increasing it in more than a quarter of them. The effects on growth were specific to particular strains and environments. These findings lead True and Lindquist to suggest that the N and M regions of Sup35 might be maintained by selection because, under conditions that are inhospitable to wild- type yeasts, cells that spontaneously switch to the prion state may by chance fare better and increase in frequency.
This is an intriguing proposal, but is open to debate. The observation that deletion of the N and M regions of Sup35 has no obvious effect, apart from a lost ability to take up the prion conformation, does not necessarily mean that they have no other function. After all, genes can often be deleted with no serious effects under laboratory conditions4, and yet are maintained by selection in nature. True and Lindquist carried out a more direct test of other possible functions of the N and M regions by measuring growth rates of strains with deletions for these regions. These deletions did influence growth, so — as the authors acknowledge — the N and M regions may well have effects beyond those mediated by their ability to take up the prion conformation.
If selection for 'evolvability' is to maintain the N and M regions of Sup35, then the prion version of the protein must increase in frequency because it occasionally creates favourable variants. Even if the chance of stumbling on such a variant is low, the consequent increase may be so large that genomes carrying the N and M regions increase on average relative to those that do not. Increased mutation or recombination rates can be favoured in the same way: a gene that modifies the genetic system gains an advantage by associating with the favourable variations that it generates. A peculiar feature of True and Lindquist's example is that variants are produced by a heritable change in protein conformation, rather than by a genetic change. So if the environment changes back, the [PSI+] strain can spontaneously revert to the original type. Similarly, Taddei et al. 5 showed that genes that raise mutation rates can hitch-hike with the favourable variants that they produce, and yet may not become fixed in the genome: back-mutation causes reversion to the original low-mutation genotype, and hence avoids the harmful consequences of permanently increased mutability. Such reversion makes selection for 'evolvability' more plausible.
These results3 show that previously hidden variation may be revealed by perturbations to the mechanism of gene expression. Other examples are well known, and have been taken as evidence that selection has shaped the organism so as to direct development towards the optimal state6-8. As True and Lindquist point out, and as many experiments have demonstrated7, 9, variants revealed by non-genetic perturbations may be picked up by selection and thus permanently incorporated into the genome. However, we feel that it is simpler to see the increased variability in these examples as a side effect of disrupted gene expression, rather than as an adaptation to facilitate evolution.
True and Lindquist's study is motivated by the idea that evolution requires "the concerted effects of several independent genetic changes", each of which is "deleterious individually"; indeed, this apparent difficulty is a frequent criticism of darwinian evolution. But it is not, as the authors state, "A major enigma in evolutionary biology": there is no evidence that the individual steps towards a complex adaptation are deleterious10. The final product may be complex and finely tuned, such that most changes are deleterious, but that does not mean that there is no path by which natural selection could construct the adaptation. The power of natural selection is that it assembles a series of changes, each individually tested; mechanisms that produce large variations, involving several random changes, are unlikely to be helpful. So we disagree with the authors over whether new mechanisms for generating radically different variants would help explain adaptive evolution.
Nonetheless, True and Lindquist's hypothesis is feasible in principle, and it is testable. To demonstrate that the N and M regions of the Sup35 protein are maintained by occasional selection for the evolutionary novelties produced by the prion, one could look in nature for a correlation between the presence of the prion and stressful environments. More practicably, one could allow laboratory strains with and without the N and M regions of Sup35 to compete in both constant and varied environments.
It is likely
that specific gene sequences have evolved to generate specific kinds of
variation11 (for example, in the vertebrate
immune system). Moreover, the genetic recombination that occurs during
sexual reproduction is likely to have evolved as an adaptation to generate
variability. Yet it is sobering that, despite several decades of intense
research, we still have little direct evidence that genetic systems have
in fact evolved to facilitate evolution2.
![]()
| 1. | Johnson, T. Genetics 151, 1621-1631 (1999). | PubMed | ISI | |
| 2. | Barton, N. H. & Charlesworth, B. Science 281, 1986-1990 (1998). | PubMed | ISI | |
| 3. | True, H. L. & Lindquist, S. L. Nature 407, 477-483 (2000). | Article | PubMed | ISI | |
| 4. | Wagner, A. Nature Genet. 24, 355-361 (2000). | Article | PubMed | ISI | |
| 5. | Taddei, F. et al. Nature 387, 700-703 (1997). | Article | PubMed | ISI | |
| 6. | Waddington, C. H. Nature 150, 563-565 (1942). |
| 7. | McLaren, A. Trends Genet. 15, 169-171 (1999). | Article | PubMed | ISI | |
| 8. | Gibson, G. & Wagner, G. Bioessays 22, 372-380 (2000). | PubMed | ISI | |
| 9. | Rutherford, S. L. & Lindquist, S. Nature 396, 336-342 (1998). | Article | PubMed | ISI | |
| 10. | Coyne, J. A., Barton, N. H. & Turelli, M. Evolution 51, 643-671 (1997). | ISI | |
| 11. | Metzgar, D. & Wills, C. Cell 101, 581-584 (2000). | PubMed | ISI | |