The Basic Structure of the Yeast Telosome. The yeast telomere nucleates
numerous multimeric complexes. The double-stranded portion of telomeric
TG1-3 tracts is bound to multiple copies of the major telomere binding
protein Rap1. Rap1 forms two major complexes; one associated with the
negative regulators of telomerase Rif1 and Rif2[1], [2], [3] and a second
associated with the heterochromatic proteins Sir3p and Sir4 (Figure 1;
[4]). In addition, the Ku 70/Ku80 heterodimer associates with the terminus
where it associates with the RNA component of telomerase[5] and is critical
for telomere size control, telomere cap function, and specific positioning
of telomeres to the nuclear periphery ([6],[7],[8],[9],[10],[11]). An
additional complex, MRX (Mre11/Rad50/Xrs2), is also important for telomere
3’ end processing and telomerase recruitment ([12], [13]).
Figure 1. The Telomere Equilibrium. The telomere is shown
adjacent to the Y’ and X subtelomeric elements in an equilibrium
between association of silencing and negative regulators of telomere size.
The Maintenance of Telomere Size: There are three basic
components of telomere size control: the telomerase holoenzyme, complementary
strand synthesis, and the maintenance of a mean telomere size at a genetically
controlled constant value.
(a) Telomerase Synthesis---Most eukaryotic telomeric DNA
is composed of simple G + T-rich sequence, following the consensus {(T/A)1-4N0-4G1-8},
with the G-rich strand oriented in a 5' to 3' direction towards the terminus
([14], [15]). In yeast, this sequence is composed of a TG1-3 irregular
repeat. The catalytic reverse transcriptase subunit (Est2p in yeast) ([16],[17])
and the telomerase RNA (TLC1 in yeast) ([14],[17]) are sufficient for
activity in vitro and define the "core" enzyme. TLC1 acts as
a template for the addition of telomeric repeats onto G-rich single-stranded
substrates both in vivo and in vitro ([18],[19],[20],[21],[22],[23]).
Telomerase activity is utilized for telomere addition in the vast majority
of eukaryotes ([15]).
The Telomerase Mechanism for Telomere Addition-Additional
components of the telomerase RNP holoenzyme are essential for activity
in vivo. One class of mutations defines genes that encode non-catalytic
components of the holotelomerase. (e.g., Est1, Est3p, and Cdc13/Est4p)
([24], [25]). First, Cdc13 bound to the elongated single-stranded overhang
in late S phase may selectively recruit Est1, which, in turn, recruits
telomerase ([25], [26] ([13]). A second set of studies suggested a cell-cycle
and Cdc13-independent binding of the core enzyme throughout the cell cycle
with S-phase specific activation of telomerase, possibly mediated through
interactions with Cdc13 and Est1 ([27], [28]). The complementary strand
is synthesized in coordination with telomerase by components of the conventional
lagging strand synthesis machinery, including Pol d Pol a, and RNA primase
([29],[30],[31]) coupled with a nucleolytic mechanism for generating a
new 3’ end ([32], [32],[33, 34],[35]).
Alternative Mechanisms for Telomere Elongation- In telomerase-negative
cells or in cells containing aberrant telomere structures (e.g., short
telomeres, unprocessed telomeric ends or overelongated 3’ overhangs),
recombination pathways allow the survival of the population. Two predominant
types of pathways have been identified. Type I recombination amplifies
subtelomeric Y’ elements ([36], [37],[38],[39]) and is dependent
on both Rad52 and Rad51, the major yeast RecA strand exchange homologue.
The underlying mechanism of Type I recombination may involve gene conversion
through either single strand annealing or strand invasion in G2/M arrested
cells during senescence ([40]).
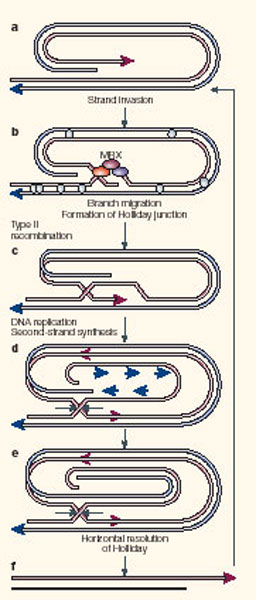
However, a more efficient pathway for survival is RAD51-independent RAD52-dependent Type II recombination between telomeric sequences ([36],[37],[38],[39]). Type II recombination may be initiated by continual cycles of replication of either an extra-chromosomal excised circle (rolling circle) or by replication through an intrachromatid t-loop intermediate (‘rolling loop’ see Figure 2) ([41],[42],[39, 43]).
This process is triggered by shortened or improperly processed
termini ([44]). Type II recombination is also dependent on Rad59, a Rad52
homologue, Sgs2, the Werner’s syndrome (WRN) helicase homologue,
and Tel1, a yeast ATM-like orthologue ([37],[38, 43]). rad51rad50 double
mutants effectively eliminate both pathways of suppressors (Chen et al
2001).
Interestingly, a break-induced replication (BIR) pathway
of DNA repair between short inverted repeats requires many of the same
gene products as Type II recombination ([45]). Remarkably, only 33 bp
of homology is required for efficient recombination ([45], [46]). These
shared requirements and the low level of homology within telomeric tracts
argue for a mechanistic relationship between short sequence recombination
and Type II recombination. (Figure 2, [43],[47]).
Telomere Length Homeostasis. In most organisms, telomeric
sequences are replicated imprecisely. Even an individual chromosomal end
varies in size among different cells of a population ([48],[49],[50]).
However, the average telomere size is tightly regulated, since tracts
in most organisms are clustered within discrete size distributions ([15],[48],[49],[50]).
In yeast, individual telomeres have tract sizes within 50 bp of a genetically
determined mean size ([14], [15]). Telomeres are subject to mechanisms
of addition that are inversely proportional to telomere size, thereby
maintaining an average telomere length. ([31],[51],[52],[53],[54]). Multiple
pathways underlie the maintenance of telomere tract size. First, the C-terminus
of yeast Rap1 appears to be counted until wild type size is attained possibly
due to the formation a thermodynamically stable structure ([51]). In contrast,
longer telomeres shorten slowly to the optimal structure due to replicative
and nucleolytic loss [52]). Second, in some contexts, Rap1 may act as
a more direct negative regulator of telomerase ([55]). This negative regulation
may be mediated through a switch or switches to an open chromatin state
conducive to telomerase access or recruitment ([55]). The third mechanism,
TRD, returns over-elongated telomeres to wild-type size through an intrachromatid
recombination process. This process, the focus of this proposal, appears
to be maintained in eukaryotes from yeast to humans, where it may act
during apoptosis and in pre-oncogenic states ([56], [57]).
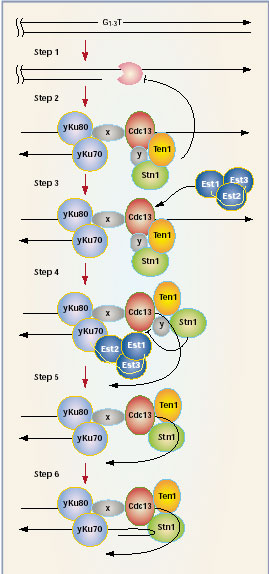
An Equilibrium between Telomerase and End Protection
Lundblad and colleagues have proposed a model for the coordination between
capping and replication. One variation of this model is depicted in Figure
3 ([31],[58]).
The C-strand of a fully replicated telomere is degraded
by an exonuclease in late S phase to give rise to a telomere containing
a >30 bp 3’ overhang. Cdc13 binds and recruits Stn1 and Ten1 to
the single stranded overhang. Both Stn1p and Cdc13 may be responsible
for the binding of Ten1p. The Ku subunits that bind to the duplex telomere
sequence may also functionally associate with Cdc13 and with TLC1 ([59];
[5]). The resulting structure would constitute the telomere end-protection
complex that represses further nucleolytic degradation. The telomerase
holoenzyme is converted to its active form and both Ku70 and Est1 associate
with TLC1 to positively regulate telomerase ([5] [60]). Stn1 subsequently
competes with Est1 for overlapping sites on Cdc13, displacing Est1 and
telomerase. Stn1 reassociates with Cdc13 directly forming a trimeric Cdc13–Stn1–Ten1
that permits second strand synthesis.
Regulating Second Strand Telomere Synthesis
Second-strand synthesis is carefully coordinated with telomerase activity
[31]. Biochemical and genetic associations have been observed between
Cdc13 and both pol a and Est1 ([61], [62]), suggesting that their recruitment
controls leading/lagging strand homeostasis[63], [64]). Furthermore, telomerase
activity on short telomere substrates in late S/G2 cells require DNA primase,
pol a, and pol d; a clear demonstration of the need for coordinated GT
and CA strand synthesis ([65]). Recent data indicate that Stn1 and the
polymerase a regulatory subunit Pol12 interact with and play an as yet
critical but undefined function in this coordination (D. Shore personal
communication).
1. Wotton, D., and Shore, D. (1997). A novel Rap1p-interacting
factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces
cerevisiae. Genes Dev 11, 748-760.
2. Hardy, C.F., Sussel, L., and Shore, D. (1992). A RAP1-interacting protein
involved in transcriptional silencing and telomere length regulation.
Genes Dev. 6, 801-814.
3. Bourns, B.D., Alexander, M.K., Smith, A.M., and Zakian, V.A. (1998).
Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in
vivo. Mol Cell Biol 18, 5600-5608.
4. Lustig, A.J. (1998). Mechanisms of silencing in Saccharomyces cerevisiae.
Curr Opin Genet Dev 8, 233-239.
5. Stellwagen, A.E., Haimberger, Z.W., Veatch, J.R., and Gottschling,
D.E. (2003). Ku interacts with telomerase RNA to promote telomere addition
at native and broken chromosome ends. Genes Dev 17, 2384-2395.
6. Lustig, A.J. (1999). The Kudos of non-homologous end-joining. Nat.
Genet. 23, 130-131.
7. Peterson, S.E., Stellwagen, A.E., Diede, S.J., Singer, M.S., Haimberger,
Z.W., Johnson, C.O., Tzoneva, M., and Gottschling, D.E. (2001). The function
of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku.
Nat Genet 27, 64-67.
8. Martin, S.G., Laroche, T., Suka, N., Grunstein, M., and Gasser, S.M.
(1999). Relocalization of telomeric Ku and SIR proteins in response to
DNA strand breaks in yeast. Cell 97, 621-633.
9. Laroche, T., Martin, S.G., Gotta, M., Gorham, H.C., Pryde, F.E., Louis,
E.J., and Gasser, S.M. (1998). Mutation of yeast Ku genes disrupts the
subnuclear organization of telomeres. Curr. Biol. 8, 653-656.
10. Gravel, S., Larrivee, M., Labrecque, P., and Wellinger, R.J. (1998).
Yeast Ku as a regulator of chromosomal DNA end structure. Science 280,
741-744.
11. Polotnianka, R.M., Li, J., and Lustig, A.J. (1998). The yeast Ku heterodimer
is essential for protection of the telomere against nucleolytic and recombinational
activities. Curr. Biol. 8, 831-834.
12. Diede, S.J., and Gottschling, D.E. (2001). Exonuclease activity is
required for sequence addition and Cdc13p loading at a de novo telomere.
Curr Biol 11, 1336-1340.
13. Tsukamoto, Y., Taggart, A.K., and Zakian, V.A. (2001). The role of
the Mre11-Rad50-Xrs2 complex in telomerase- mediated lengthening of Saccharomyces
cerevisiae telomeres. Curr Biol 11, 1328-1335.
14. Greider, C.W. (1996). Telomere length regulation. Annu Rev Biochem
65, 337-365.
15. Zakian, V.A. (1996). Structure, function, and replication of Saccharomyces
cerevisiae telomeres. Annu Rev Genet 30, 141-172.
16. Lingner, J., and Cech, T.R. (1996). Purification of telomerase from
Euplotes aediculatus: requirement of a primer 3' overhang. Proc Natl Acad
Sci U S A 93, 10712-10717.
17. Lingner, J., Hughes, T.R., Shevchenko, A., Mann, M., Lundblad, V.,
and Cech, T.R. (1997). Reverse transcriptase motifs in the catalytic subunit
of telomerase. Science 276, 561-567.
18. Wen, J., Cong, Y.S., and Bacchetti, S. (1998). Reconstitution of wild-type
or mutant telomerase activity in telomerase- negative immortal human cells.
Hum Mol Genet 7, 1137-1141.
19. Beattie, T.L., Zhou, W., Robinson, M.O., and Harrington, L. (1998).
Reconstitution of human telomerase activity in vitro. Curr Biol 8, 177-180.
20. Lingner, J., Cech, T.R., Hughes, T.R., and Lundblad, V. (1997). Three
Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase
activity. Proc. Natl. Acad. Sci. U. S. A. 94, 11190-11195.
21. Greider, C.W., and Blackburn, E.H. (1989). A telomeric sequence in
the RNA of Tetrahymena telomerase required for telomere repeat synthesis.
Nature 337, 331-337.
22. McEachern, M.J., and Blackburn, E.H. (1996). Cap-prevented recombination
between terminal repeat arrays (telomere CPR) maintains telomeres in Kluyvermyces
lactis lacking telomerase. Gene 11, 528-540.
23. Prescott, J., and Blackburn, E.H. (1997). Telomerase RNA mutations
in Saccharomyces cerevisiae alter telomerase action and reveal nonprocessivity
in vivo and in vitro. Gene 11, 528-540.
24. Virta-Pearlman, V., Morris, D.K., and Lundblad, V. (1996). Est1 has
the properties of a single-stranded telomere end-binding protein. Genes
Dev 10, 3094-3104.
25. Evans, S.K., and Lundblad, V. (1999). Est1 and Cdc13 as comediators
of telomerase access. Science 286, 117-120.
26. Pennock, E., Buckley, K., and Lundblad, V. (2001). Cdc13 delivers
separate complexes to the telomere for end protection and replication.
Cell 104, 387-396.
27. Steiner, B.R., Hidaka, K., and Futcher, B. (1996). Association of
the Est1 protein with telomerase activity in yeast. Proc Natl Acad Sci
U S A 93, 2817-2821.
28. Hughes, T.R., Evans, S.K., Weilbaecher, R.G., and Lundblad, V. (2000).
The Est3 protein is a subunit of yeast telomerase. Curr Biol 10, 809-812.
29. Dionne, I., and Wellinger, R.J. (1998). Processing of telomeric DNA
ends requires the passage of a replication fork. Nucleic Acids Res 26,
5365-5371.
30. Adams, A.K., and Holm, C. (1996). Specific DNA replication mutations
affect telomere length in Saccharomyces cerevisiae. Mol Cell Biol 16,
4614-4620.
31. Adams Martin, A., Dionne, I., Wellinger, R.J., and Holm, C. (2000).
The function of DNA polymerase alpha at telomeric G tails is important
for telomere homeostasis. Mol Cell Biol 20, 786-796.
32. McElligott, R., and Wellinger, R.J. (1997). The terminal DNA structure
of mammalian chromosomes. Embo J 16, 3705-3714.
33. Wright, W.E., Tesmer, V.M., Huffman, K.E., Levene, S.D., and Shay,
J.W. (1997). Normal human chromosomes have long G-rich telomeric overhangs
at one end. Genes Dev 11, 2801-2809.
34. Li, L., Makarov, S., and Langmore, J.P. (1998). In vitro and in vivo
reconstitution and stability of vertebrate chromosomes ends. Nucleic Acids
Res. 26, 2908-2916.
35. Dionne, I., and Wellinger, R.J. (1996). Cell cycle-regulated generation
of single-stranded G-rich DNA in the absence of telomerase. Proc Natl
Acad Sci U S A 93, 13902-13907.
36. Lundblad, V., and Blackburn, E.H. (1993). An alternative pathway for
yeast telomere maintenance rescues est1- senescence. Cell 73, 347-360.
37. Teng, S.C., and Zakian, V.A. (1999). Telomere-telomere recombination
is an efficient bypass pathway for telomere maintenance in Saccharomyces
cerevisiae. Mol Cell Biol 19, 8083-8093.
38. Teng, S., Chang, J., McCowan, B., and Zakian, V.A. (2000). Telomerase-independent
lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, rif-inhibited
recombinational process. Mol. Cell. 6, 947-952.
39. Le, S., Moore, J.K., Haber, J.E., and Greider, C.W. (1999). RAD50
and RAD51 define two pathways that collaborate to maintain telomeres in
the absence of telomerase. Genetics 152, 143-152.
40. Signon, L., Malkova, A., Naylor, M.L., Klein, H., and Haber, J.E.
(2001). Genetic requirements for RAD51- and RAD54-independent break-induced
replication repair of a chromosomal double-strand break. Mol Cell Biol
21, 2048-2056.
41. McEachern, M.J., and Iyer, S. (2001). Short telomeres in yeast are
highly recombinogenic. Mol Cell 7, 695-704.
42. Natarajan, S., and McEachern, M.J. (2002). Recombinational telomere
elongation promoted by DNA circles. Mol Cell Biol 22, 4512-4521.
43. Chen, Q., Ijpma, A., and Greider, C.W. (2001). Two survivor pathways
that allow growth in the absence of telomerase are generated by distinct
telomere recombination events. Mol Cell Biol 21, 1819-1827.
44. Grandin, N., Damon, C., and Charbonneau, M. (2001). Cdc13 prevents
telomere uncapping and Rad50-dependent homologous recombination. Embo
J 20, 6127-6139.
45. Ira, G., and Haber, J.E. (2002). Characterization of RAD51-independent
break-induced replication that acts preferentially with short homologous
sequences. Mol Cell Biol 22, 6384-6392.
46. Manthey, G.M., and Bailis, A.M. (2002). Multiple pathways promote
short-sequence recombination in Saccharomyces cerevisiae. Mol Cell Biol
22, 5347-5356.
47. Bucholc, M., Park, Y., and Lustig, A.J. (2001). Intrachromatid excision
of telomeric DNA as a mechanism for telomere size control in Saccharomyces
cerevisiae. Mol Cell Biol 21, 6559-6573.
48. Walmsley, R.M., and Petes, T.D. (1985). Genetic control of chromosome
length in yeast. Proc. Natl. Acad. Sci. U. S. A. 82, 506-510.
49. Shampay, J., and Blackburn, E.H. (1988). Generation of telomere-length
heterogeneity in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S.
A. 85, 534-538.
50. Smogorzewska, A., van Steensel, B., Bianchi, A., Oelmann, S., Schaefer,
M.R., Schnapp, G., and de Lange, T. (2000). Control of human telomere
length by TRF1 and TRF2. Mol. Cell. Biol. 20, 1659-1668.
51. Marcand, S., Gilson, E., and Shore, D. (1997). A protein-counting
mechanism for telomere length regulation in yeast. Science 275, 986-990.
52. Marcand, S., Brevet, V., and Gilson, E. (1999). Progressive cis-inhibition
of telomerase upon telomere elongation. EMBO J. 18, 3509-3519.
53. van Steensel, B., and de Lange, T. (1997). Control of telomere length
by the human telomeric protein TRF1. Nature 385, 740-743.
54. Ray, A., and Runge, K.W. (1999). The yeast telomere length counting
machinery is sensitive to sequences at the telomere-nontelomere junction.
Mol. Cell. Biol. 19, 31-45.
55. Prescott, J.C., and Blackburn, E.H. (2000). Telomerase RNA template
mutations reveal sequence-specific requirements for the activation and
repression of telomerase action at telomeres. Mol Cell Biol 20, 2941-2948.
56. Ramirez, R., Carracedo, J., Jimenez, R., Canela, A., Herrera, E.,
Aljama, P., and Blasco, M.A. (2003). Massive Telomere Loss Is an Early
Event of DNA Damage-induced Apoptosis. J Biol Chem 278, 836-842.
57. Baird, D., Rowson, J., Wynford-Thomas, D., and Kipling, D. (2003).
Extensive allelic variation and ultrashort telomeres in senescent human
cells. Nature Genetics 33, 203-207.
58. Lustig, A.J. (2001). Cdc13 subcomplexes regulate multiple telomere
functions. Nat. Struct. Biol. 8, 297-299.
59. Evans, S.K., and Lundblad, V. (2002). The Est1 Subunit of Saccharomyces
cerevisiae Telomerase Makes Multiple Contributions to Telomere Length
Maintenance. Genetics 162, 1101-1115.
60. Livengood, A.J., Zaug, A.J., and Cech, T.R. (2002). Essential regions
of Saccharomyces cerevisiae telomerase RNA: separate elements for Est1p
and Est2p interaction. Mol Cell Biol 22, 2366-2374.
61. Qi, H., and Zakian, V.A. (2000). The Saccharomyces telomere-binding
protein Cdc13p interacts with both the catalytic subunit of DNA polymerase
alpha and the telomerase-associated est1 protein. Genes Dev 14, 1777-1788.
62. Chandra, A., Hughes, T.R., Nugent, C.I., and Lundblad, V. (2001).
Cdc13 both positively and negatively regulates telomere replication. Genes
Dev. 15, 404-414.
63. Qi, H., and Zakian, V.A. (2000). The Saccharomyces telomere-binding
protein Cdc13p interacts with both the catalytic subunit of DNA polymerase
alpha and the telomerase-associated E1 protein. Genes Dev. 14, 1777-1788.
64. Pennock, E., Buckley, K., and Lundblad, V. (2001). Cdc13 delivers
separate complexes to the telomere for end protection and replication.
Cell 104, 387-396.
65. Diede, S.J., and Gottschling, D.E. (1999). Telomerase-mediated telomere
addition in vivo requires DNA primase and DNA polymerases alpha and delta.
Cell 99, 723-733.