
|
Our laboratory conducts experimental and computational research in biomedical acoustics (therapeutic and diagnostic ultrasound) and biomechanics of circulating cells. Using in vitro, ex vivo and in vivo models, we investigate mechanochemical disrpution of living cells and tissues induced by high-intensity focused ultrasound (HIFU) and sensitizing chemical agents, with the goal to develop minimally invasive therapy that can stop cancer progression to its lethal stage. The recent data of our experiments indicate that mechanochemical disruption breaks drug resistance of tumor cells and reprograms them to a healthier, non-metastatic phenotype. We also study the stimulatory effects of low-intensity ultrasound on axonal growth in cortical neurons to develop better therapies for patients with spinal cord injury and other neurological deficits. Our diagnostic ultrasound research focuses on small-volume noncontact blood coagulation analysis of neonatal, pediatric and adult patients by our patented acoustic tweezing method. 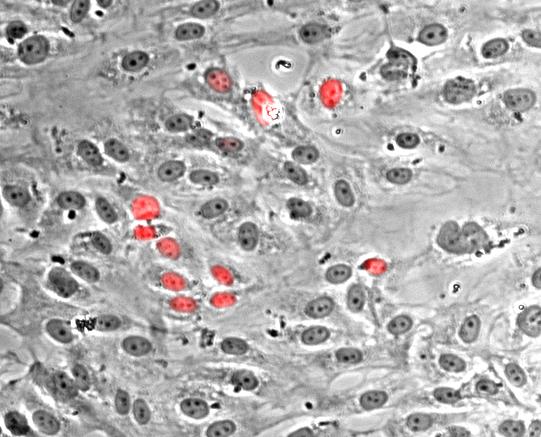
Nuclei of poliferating endothelial cells stained with Hoechst dye (red)
Using endothelium-lined microfluidic systems and state-of-the-art computational models, we study migration, deformation and adhesion of circulating cells as well as endothelial dysfunction under the
conditions of cancer metastasis, inflammation and cardiovascular disease. |
Lab highlights
|
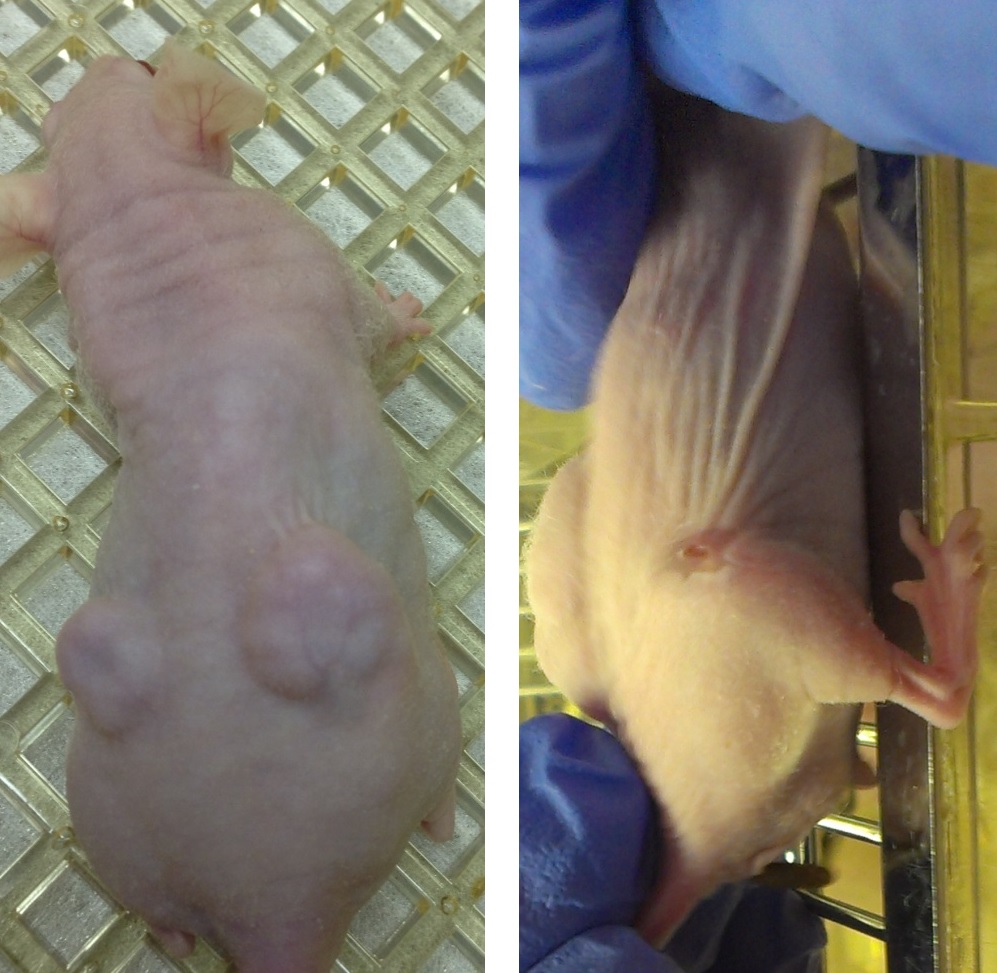 |
Our recent experiments show that the exposure of tumors and cancer cells (prostate, liver) to a combination of ethanol injection and HIFU leads to dramatic tumor regression and a significant decrease in 1) cell viability and proliferation, 2) expression of metastatic markers, 3) adhesion to vascular endothelium, 4) cell migratory ability, and 5) tumorigenic potential. We also have very promising data on breaking drug resistance of breast cancer cells by HIFU.
Acoustic tweezing thromboelastometry: a novel and robust technique for low-volume coagulation analysis of whole blood and blood plasma >>
published data in Journal of Thrombosis and Haemostatsis, NSF I-Corps grant awarded... |
We have patented a non-contact method and device for rheological measurements of polymeric and biological fluids, referred to as "acoustic tweezing rheometry" or "acoustic tweezing thromboelastometry". Our first clinical studies clearly show the ability of this technique to detect the onset times for blood coagulation and mature blood clot formation and to measure the clot stiffness for normal blood and blood exposed to pro- and anti-coagulants. We began commercializing our technology for coagulation measurement of neonatal and pediatric patients as well as adult patients with coagulopathy.
Oxidized lipoproteins cause endothelial dysfunction via activation of tissue-resident cells >>
supported by a NIH R01 grant...Using our endothelium-lined microfluidic channels, we investigate the role of inflammatory mediators produced by tissue resident cells on circulating cell adhesion to vascular or lymphatic endothelium during allergy, atherosclerosis, thrombosis, sickle cell disease, and cancer metastasis. Our recent data point out an important synergistic role of oxidized lipoprotein-activated macrophages and mast cells in endothelial dysfunction and early atherosclerosis.
VECAM-Active is the first computational algorithm to simulate active migration of motile cells in 3-D >>
unique model for chemotactic and haptotactic migration of cells on a deformable substrate or inside a viscoelastic tissue...We have developed a three-dimensional (3-D) computational algorithm, known as VECAM (ViscoElastic Cell Adhesion Model). VECAM integrates, for the first time, the cell's rheological properties, stochastic receptor-ligand binding, and physiologic shear flow conditions. Recently, this algorithm has been extended (VECAM-Active) to simulate chemoattractant-induced active migration of motile cells in 3-D or on an adhesive 2-D substrate. Using VECAM-Active, we now explore the mechanisms that govern chemotaxis, adhesion, and transendothelial migration of leukocytes and circulating tumor cells, which are key steps of the inflammatory response and cancer metastasis.