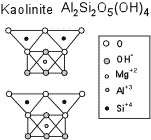
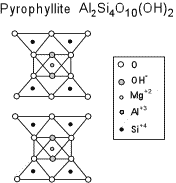
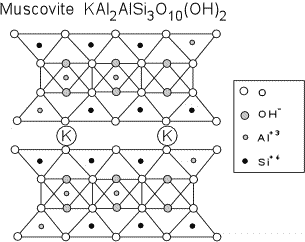
Distinguishing Clay Minerals
Generally, the clay minerals occur as such small mineral grains that
they cannot be easily distinguished in either hand specimen or thin
section. However, the smectites can be distinguished from the other
clays in the field by the "eating test" - place some clay in
your mouth. If you can feel it expand as it becomes moistened, then
it is one of the smectite clays, and is not a kandite or illite clay.
X-ray techniques, are thus usually required to identify the clay
minerals. First, however, the clays have to be separated from other constituents.
To do this, we first disaggregate the sample and place it in a settling
tube filled with water. Particles will settle in the water according
to Stokes Law:
V = 2/9(ρg -ρw)
g r2/η
where
V = the settling velocity
ρg = density of the mineral
grain (2.6 - 2.8 g/cm3 for clay minerals)
ρw = density of water (1g/cm3)
g = acceleration due to gravity (980 cm/sec2)
r = radius of the mineral particle (10-4 cm
for clays)
η = viscosity of water (10-2
gcm/sec2)
Usually a desegregating agent (Calgon) is added to the water to keep
the individual particles from adhering to one another. The particles
are placed in a large glass cylinder filled with water and the
desegregating agent, and the mixture is stirred.
One then must use Stokes law to figure out how far particles of clay
size will settle in a given time. That distance is measured on the
cylinder, and that amount of water is then poured off and collected.
It is then poured through a filter to separate the clay minerals from the
water. The filter is then dried and the clay minerals are placed on
a glass slide ready for X-ray diffraction analysis.
Recall that Bragg's Law:
nλ = 2d sin θ
allows one to calculate the "d" spacing between lattice
planes if the wavelength, λ, of the X-rays is
known, and the diffraction angle θ is
known.
Normally in powder X-ray diffraction studies we would want the mineral
grains to be oriented randomly on the glass slide. But for clay
minerals, the most diagnostic "d" spacing is between the {001}
planes. So, when the grains are placed on the glass slide they are usually
placed in a few drops of water so that they will settle onto the slide
with their {001} planes parallel to the slide. Thus, when we X-ray
them, we get diffraction predominantly off of the {001} planes and can
measure the "d" spacing between these planes.
The table below shows the d spacing for the {001} plane as measured for
various clay-type minerals. Untreated is for the minerals in their
natural state, Ethylene Glycol values are obtained after treating the
minerals in a solution of Ethylene Glycol (the principal ingredient in
anti-freeze), and the last column show the effect if the mineral is heated
to 550oC after the Ethylene Glycol treatment. |