| Geology 212 | Petrology |
Prof. Stephen A. Nelson |
Carbonates & Other Rocks |
||
Carbonate Rocks The carbonate rocks make up 10 to 15% of sedimentary rocks. They largely consist of two types of rocks.
Because carbonate minerals in general are soluble in slightly acidic waters, they often have high porosity and permeability, making them ideal reservoirs for petroleum. For this reason they are well studied. Limestone can be easily recognized in hand specimen or outcrop because of its high solubility in HCl. A drop of such acid placed on the rock will cause it to fizz due to the generation of CO2 gas. A dolostone, on the other hand, will not fizz until a fine powder is made from the rock or mineral. Also, dolostones tend to weather to a brownish color rock, whereas limestones tend to weather to a white or gray colored rock. The brown color of dolostones is due to the fact that Fe occurs in small amounts replacing some of the Mg in dolomite. |
| Classification |
|
|
|
|
| Textures |
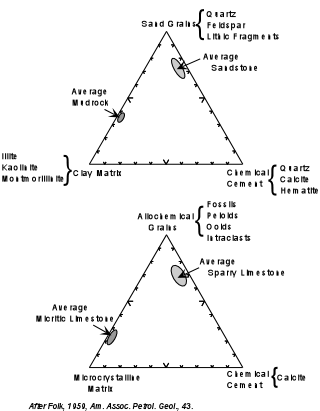
| Many limestones (carbonate rocks in general) show characteristics similar
to those of clastic sediments, like sandstones. Sandstones are composed of sand
grains, a mud or clay matrix, and a crystalline cement produced during diagenesis.
Similarly carbonate rocks are composed of allochemical grains (grains produced by
precipitation somewhere else and transported, usually short distances, to the depositional
site), mud matrix, consisting of fine-grained carbonate minerals, and a crystalline cement
of calcite (or dolomite) precipitated during diagenesis. From the figure shown here,
one can see that the average sandstone and mudrock have similar proportions of analogous
constituents to average sparry (crystalline) limestones and micritic (fine grained
crystalline limestones. This suggests a similarity of processes involved in the
formation of clastic sediments and carbonate rocks.
|
 |
|
|
|
| Structures
|
|
|
 |
| Carbonate Depositional Environments
|
| Dolostones Dolostones are carbonate rocks composed almost entirely of dolomite - (Ca,Mg)CO3. Although there used to be a common perception that the abundance of dolostones increased with age of the rock, it is now recognized that although no primary dolomite bearing rocks are being directly precipitated in modern times, dolostones have formed throughout geologic time. This is true despite the fact that modern sea water is saturated with respect to dolomite. Still, most dolostones appear to result from diagenetic conversion of calcite or high-Mg calcite to dolomite, after primary deposition of the original calcium carbonate bearing minerals. Dolomite, and therefore rocks containing large amounts of dolomite, like dolostones, is easily distinguished by the fact that it only fizzes in dilute HCl if broken down to a fine powder. Also, dolostones tend to weather to a brownish color rock, whereas limestones tend to weather to a white or gray colored rock. The brown color of dolostones is due to the fact that Fe occurs in small amounts replacing some of the Mg in dolomite. In thin section it is more difficult to distinguish from calcite, unless it is twined. Unfortunately, sedimentary dolomite is rarely twinned. In order to facilitate its identification in thin section, the sections are often stained with alizarin red S. This turns calcite pink, but leaves the dolomite unstained. Dolostones are almost entirely composed of euhedral and subhedral rhombs of dolomite. Although dolostones contain allochems, like limestones, the allochems are generally recrystallized to dolomite, and rhombs of dolomite can be seen to cut across the boundaries of allochemical particles. Some dolostones show no evidence of allochems, but only contain rhombs of dolomite. These could either represent limestones that have completely recrystallized to dolomite, leaving no trace of the original fragments that made up the limestone, or could represent primary crystals of dolomite. Two mechanisms of dolomitization of limestones have been proposed based on field and laboratory studies.
|
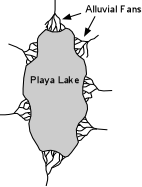
Other Sedimentary Rocks Evaporites |
|
 |
|
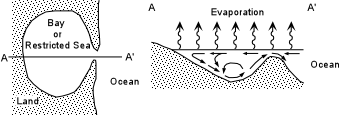 |
These dense saline waters then sink within the basin, become oversaturated with respect
to salts like gypsum and halite, and precipitate the salts on the floor of the basin. |
|
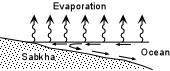 |
Cherts Chert is a mineralogically simple rock consisting of microcrystalline quartz. There are three common occurrences of chert.
|
Since mechanisms 1 and 2 generally require the presence of silica secreting organisms in order to form chert, the occurrence of chert in Precambrian rocks is problematical because no such organisms existed prior to the early Paleozoic. Such Precambrian cherts may have actually formed by direct chemical precipitation from silica oversaturated seawater. |
| Return to Geology 212 Home Page |