|
The majority of protozoan species are free living and have little impact on human health. Free-living protozoa can be found throughout the environment and are particularly abundant in soil and water. One example of free-living protozoa affecting human health are some free-living amebae which can cause pathology if introduced into the human host. Another means by which some free-living protozoa can affect humans is indirectly through their effect on the environment. For example, some free-living protozoa produce toxins which can cause disease (eg., red tide).
Free-living amebae known to cause human disease are:
| • | Naegleria fowleri | acute primary amebic meningoencephalitis (PAM) |
| • | Acanthamoeba spp. | chronic granulomatous amebic encephalitis (GAE); amebic keratitis; granulomatous skin and lung lesions |
| • | Balamuthia mandrillaris | sub-acute to chronic GAE; granulomatous skin and lung lesions |
| • | Sappinia diploidea | single case report of encephalitis (Gelman et al, 2001) |
These infections are quite rare and are presumably acquired directly from the environment (eg., water or soil contact). No human-to-human transmission or vector transmission have been documented.
Naegleria species are found in freshwater habitats and moist soil throughout the world. Primary amebic meningoencephalitis (PAM) was first recognized by Fowler in 1965 and N. fowleri is the only Naegleria species known to be pathogenic to humans. As of 1996, approximately 175 cases have been reported worldwide and 81 of these are in the U.S.. Between 2001 and 2010 32 cases have been reported in the U.S.; 30 of which have been associated with recreational water. Two cases in Louisiana have been reported due to nasal irrigation with neti-pots (see http://new.dhh.louisiana.gov/index.cfm/newsroom/detail/2332).
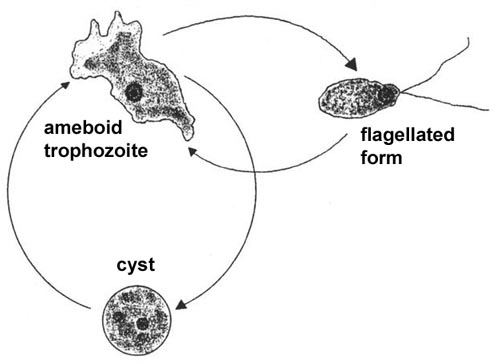
The life cycle consists of trophozoite and cyst stages and the trophozoite stage can be either ameboid or flagellated (Figure). The ameboid trophozoite feeds on bacteria and other organic matter and undergoes asexual replication. The ameboid form transforms into a pear-shaped flagellated form with two flagella at the broad end when placed in distilled water or deprived of nutrients. Flagellated trophozoites are non-feeding and do not replicate, but will convert back into the ameboid form when nutrients are restored. The ameboid forms can also encyst resulting in a stage resistant to desiccation. All stages are characterized by a single nucleus with a large karyosome and no peripheral chromatin. Only the ameboid form is found in tissue.
 |
|
||
| A typical protozoan life cycle consists of cysts and trophozoites. Trophozoites are active forms which acquire nutrients, undergo replication and are generally motile. When conditions are harsh (eg., desiccation or low nutrients) the trophozoites will secrete a thick wall and enter into a dormant period characterized by low metabolic activity. The cysts will convert back into trophozoites when conditions are favorable. Many intestinal protozoa exhibit a similar life cycle (see fecal-oral transmission). Only the ameboid trophozoite of Naegleria is associated with primary amebic meningoencephalitis (PAM). | |||
Naegleria produces a fulminant, and almost always fatal, acute meningoencephalitis in children and young adults who were previously in excellent health (see PAM Box). Almost all reported cases have been associated with swimming in warm or heated waters a few days prior to the onset of symptoms. The portal of entry appears to be the olfactory neuroepithelium in the nasal cavity. The trophozoites probably migrate along the olfactory nerves into the brain and CNS. The symptoms of PAM resemble bacterial meningoencephalitis and PAM is generally characterized by a sudden onset of headache and fever. Nausea, vomiting and other symptoms related to increase intracranial pressure may also be evident. There is a rapid progression from headache and fever to coma, with occasional seizures, and death. Diagnosis is almost always post-mortem and the prognosis is not good.
|
Acanthamoeba species are ubiquitous in soil and water. Their pathogenic potential was first recognized in 1958 by Culbertson who produced an encephalitis in mice following inoculation with an Acanthamoeba-contaminated cell culture. The first definitive human cases were reported in the early 1970's. In contrast to PAM, Acanthamoeba infections are usually associated with chronically ill, immunocompromised, or other debilitated patients. Acanthamoeba, like Naegleria, is neurotropic and causes an encephalitis like disease. However the disease, known as granulomatous amebic encephalitis (GAE), is more slowly progressing and chronic. Acanthamoeba infections are also associated with lesions in the cornea (amebic keratitis), lungs and skin.
The portal of entry for Acanthamoeba is not known but believed to the either the respiratory tract via inhalation of cysts or through wounds in the skin that become contaminated by soil. Presumably the trophozoites disseminate by a hematogenous route (i.e., via the circulatory system) to the central nervous system (CNS). However, Recavarren-Arce et al, (1999) have suggested a perivascular route of dissemination (see Box and discussion of Balamuthia). In other words, the amebas crawl along the outside of the blood vessels to reach the CNS. The onset of symptoms is often insidious and the clinical manifestations include subtle headache, personality changes and slight fever. GAE has a prolonged clinical course and can take weeks to months to progress to coma and death. Diagnosis is difficult and usually done at autopsy.
Acanthamoeba exibits a typical protozoan life cycle consisting of an ameboid trophozoite stage and a cyst stage (see figure legend of Naegleria life cycle for explanation of trophozoites and cysts). In contrast to Naegleria, both cyst and trophozoite stages can be found in histological specimens. The cysts have a three-layered wall, a wrinkled appearance and are extremely resistant to desiccation. In histological preparations the trophozoites of Acanthamoeba are very similar to Naegleria trophozoites and cannot be distinguished on morphological criteria. However, in culture Acanthamoeba trophozoites can be distinguished by their spike-like pseudopodia (Figure).
| Morphology of Free-Living Ameba | ||||||
|---|---|---|---|---|---|---|
|
||||||
| Amebic Keratitis |
|---|
|
Amebic keratitis is a vision-threatening chronic inflammation of the cornea caused by Acanthamoeba. It was first reported in 1973 and until the mid-1980's was exceedingly rare and usually associated with ocular trama. The number of cases has increased since 1985 and it is estimated that there has been more than 700 cases as of 1996. This increase is attributed to the use of contact lenses and especially incomplete cleaning and disinfection.
Acanthamoeba probably gains access to the corneal stroma through small breaks in the corneal epithelium produced as a result of minor trauma or abrasion. The ameba can then further destroy the corneal stroma or permit secondary bacterial infections. A mild to moderate corneal inflammation is also associated with Acanthamoeba invasion. Clinical manifestations of amebic keratitis include severe ocular pain and corneal lesions refractory to antiviral, antibacterial, and antimycotic drugs. Diagnosis is confirmed by detecting the amebas in corneal scrapings or biopsies. Some success in treating amebic keratitis has been obtained with poly-hexa-methylene biguanide or propamidine isethionate (Brolene). Surgery is often needed to correct the loss of vision.
| Recavarren-Arce et al (1999) |
|---|
|
Balamuthia mandrilaris is another free-living ameba capable of causing human disease. It was first reported in a mandrill baboon in 1990 and subsequently shown to be associated with human disease. The trophozoites and cysts of Balamuthia are morphologically similar to Acanthamoeba and it also causes GAE (see GAE Box). Phylogenetic analysis of rRNA sequences indicate that Balamuthia is a sister genus of Acanthamoeba and thus the two are closely related (Booton et al, 2003). But they are likely independent and monophyletic genera.
Many cases of GAE originally ascribed to Acanthamoeba have retrospectively been assigned to Balamuthia. As of 2003 there have been approximately 100 cases of Balamuthia infections reported with about half of the cases being in the U.S.. Many of the reported cases involve children. In contrast to Acanthamoeba, Balamuthia can cause a subacute-to-chronic infection which is more rapidly progressing. In addition, Balamuthia appears more capable of infecting healthy individuals (see Box). In many of the infections a primary lesions in the respiratory tract or skin have been noted suggesting that the infections is acquired via inhalation or breaks in the skin. Thus far, only 3 survivors have been reported (Deetz et al, 2003; Jung et al, 2004). All were treated for an extensive time period with numerous drugs.
Only recently has Balamuthia been identified in the environment. The amebas were successfully grown from soil samples associated with a fatal case in a northern California child (Schuster et al, 2003) and have subsequently been cultivated from another soil source (Dunnebacke et al, 2004). The ameba are slow growing (generation time of 20-50 hours) and feed on other ameba (or cultivated mammalian cells) instead of bacteria. These features probably account for the difficulty in detecting Balamuthia in the environment. Interestingly, in two of the survivors, acquisition of the infection was associated with gardening activities (Deetz et al, 2003; Jung et al, 2004).
For a review of free-living ameba see: GS Visvesvara, H Moura, FL Schuster (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunology & Medical Microbiology 2007 50: 1-26.
 |
| (Upper panel) Picture of a red tide from D. Anderson (1994). (Lower panel) Light micrograph of G. toxicus provided by Tracy Villareal (U. Texas Marine Sciences Institute). |
'Red tides', or algal blooms, are the result of large increases in the number of unicellular planktonic organisms. Dinoflagellates--claimed by protozoologists as protozoa and by phycologists as algae--are a major component of the microscopic zoo- and phytoplankton. Many of the dinoflagellates contain pigments and therefore will result in the water taking on a distinct colored appearance in the affected areas (usually coastal). Many dinoflagellates are colorless, but nonetheless, fall under the rubric of red tides. Recent increases in algal blooms are usually attributed to higher levels of nutrients in estuaries and coastal waters due to pollution and agricultural runoff. The shipping industry has also been blamed for increase worldwide distribution of organisms causing red tides.
An overabundance of metabolically-active microorganisms can cause 'dead zones' by depleting the water of oxygen. Fish and other macroscopic organisms will either die or avoid these dead zones, and therefore, can have an adverse economic impact on fishermen and the seafood industry. In some cases, though, the fish kills are the result of toxins produced by dinoflagellates.
These toxins can also accumulate in fatty tissues or organs (eg., liver) and pass up the food chain and be consumed by humans. Ciguatera and shellfish poisoning are examples of human disease caused by toxic dinoflagellates (see Table below). Ciguatera--known in tropics for centuries and becoming more common elsewhere as tropical fish are more widely marketed--is a type of food poisoning associated with the accumulation of dinoflagellate toxins in fish. Herbivorous fish ingest toxic dinoflagellates (primarily Gambierdiscus toxicus) while feeding on seaweed and these fish are then eaten by larger fish. Thus, these larger and more desirable fish, such as barracuda, grouper, jack and snapper, will accumulate toxins over time. Some of the fish will accumulate sufficient toxin levels to cause gastrointestinal, neurological, and/or cardiovascular symptoms in humans after a single meal. The occurrence of toxic fish is sporadic and not all fish of a given species or from a ciguatera endemic region will be toxic. The major ciguatoxins have been identified as a family of cyclic polyethers which are heat-stable and lipid-soluble and activate voltage-sensitive sodium channels at nanomolar and picomolar concentrations.
Initially the symptoms may resemble food poisoning (eg., nausea, vomiting, diarrhea) occurring within 6 hours of eating a toxic fish. Initial symptoms can also include numbness and tingling around the mouth which can spread to extremities. Neurological manifestations include: intensified tingling (paresthesia), headache, pain in muscles and joints, acute sensitivity to temperature extremes, vertigo, and muscular weakness. Typical cardiovascular manifestations are arrhythmias and reduced blood pressure. Ciguatera poisoning is usually self-limiting and the symptoms typically subside within several days. However, in severe cases the neurological symptoms can persist for weeks to months, and in rare cases, neurological symptoms have persisted for years. There have been a few deaths due to respiratory or cardiovascular failures.
[For a reviews on ciguatera see: Lewis, RJ (2006) Ciguatera: Australian perspectives on a global problem. Toxicon 48: 799-809 and Ciguatera Lecture Notes]
| Examples of Toxic Dinoflagellates | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
Similarly, shellfish, which are less sensitive to algal toxins, will ingest the dinoflagellates or other algae and retain the toxins. In some cases the specific dinoflagellate as well as the toxins are known (see Table). The clinical outcome depends on the specific dinoflagellate species and are typically classified as diarrheic (DSP), paralytic (PSP), neurotoxic (NSP) or amnesic (ASP). ASP is caused by domoic acid which is produced by the diatom Pseudo-nitzschia (previously Nitzschia). Developed countries typically monitor shellfish beds and other resources for toxic algae as a means to protect people from the consumption of toxic shellfish. (See ARNAT for more information on algal toxins.)
Shellfish poisoning exhibits
a variety of symptoms depending on the exact toxin consumed and the dose. Diagnosis
is based on recent dietary history and symptoms. Salient features of the different
types of shellfish poisoining are:
| DSP | DSP is primarily observed as a mild gastrointestinal disorder (i.e., nausea, vomiting, diarrhea, and abdominal pain) accompanied by chills, headache, and fever. Onset of the disease may be as little as 30 minutes and up to 2 to 3 hours after ingestion. Symptoms may last 2 to 3 days and recovery is usually complete with no after effects. | |
| PSP | Symptoms of the disease develop within 0.5 to 2 hours after ingestion of the shellfish. They are predominantly neurological (include: tingling, burning, numbness, drowsiness, incoherent speech, and respiratory paralysis). Respiratory paralysis is observed in severe cases and death may occur if respiratory support is not provided. Recovery usually is complete with no lasting side effects if such support is applied within 12 hours of exposure. | |
| NSP | Both gastrointestinal and neurological symptoms characterize NSP (including tingling and numbness of lips, tongue, and throat, muscular aches, dizziness, reversal of the sensations of hot and cold, diarrhea, and vomiting). Onset occurs within a few minutes to a few hours after ingestion. Duration is from a few hours to several days and recovery is complete with few after effects. No fatalities have been reported. | |
| ASP | Symptoms include gastrointestinal disorders (vomiting, diarrhea, abdominal pain) and neurological problems (confusion, memory loss, disorientation, seizure, coma). Gastrointestinal symptoms occur within 24 hours and the neurological symptoms occur within 48 hours of ingestion. It is particularly serious in elderly patients and the symptoms are reminiscent of Alzheimer's disease. All fatalities to date have involved elderly patients. |
[For review on ciguatera and shellfish poisoning see The Harmful Algae.]
Pfiesteria and Possible Estuary-Associated Syndrome
Massive fish kills and reports from fishermen and swimmers complaining of rashes, respiratory problems and neurological phenomenon in North Carolina estuaries and the Chesapeake Bay during the late 1980's and early 1990's led to the identification of a previously undescribed dinoflagellate. This new dinoflagellate was assigned to a new genus Pfiesteria and two species, P. piscicida and P. shumwayae have been described. Large blooms of Pfiesteria were associated with fish kills and human health problems. The Center for Disease Control (CDC) designated these symptoms as possible estuary-associated syndrome. In contrast to shellfish poisoning or ciguatera, the adverse affects on humans and fish are thought to be through contact with the organism or toxins instead of ingestion of the toxins.
Originally, Pfiesteria was described as having a very complex life cycle involving up to 24 different morphological forms, including several types of ameboid forms. However, more recently it has been suggested that Pfiesteria has a more conventional dinoflagellate type of life cycle involving cysts and flagellated forms known as dinospores (or zoospores). The typical dinoflagellate sexual and asexual cycles are also observed.
The identification of a presumptive toxin associated with the fish killing activity has been elusive. Filtrates from Pfiesteria cultures were shown to induce open ulcerative sores, hemorrhaging and death in finfish and shellfish. However, no specific toxin could be isolated from the filtrates. It has also been proposed that Pfiesteria can interconvert between non-toxic and toxic forms in the presence of fish. Others have shown that the toxicity to fish depends on direct contact between the dinospores and the fish. Furthermore, the dinospores swarm towards the fish and attach directly to them and actively feed on the fish (see Vogelbein et al, 2002, Nature 418:967). Therefore, the killing activity may be due to micropredation and not an exotoxin secreted by the Pfiesteria.
| Symptoms Due To Exposure To Pfiesteria Aerosols |
|---|
|
The best evidence for a
toxin comes from human exposure to Pfiesteria cultures. Several investigators
have experienced neurological symptoms after being exposed to aerosols derived
from cultures. These have included both dramatic and rapid neurological effects
and long-term and chronic effects (Box). The controversies associated with the
toxicity and life cycle of Pfiesteria are due in part to the working
with environmental samples containing a variety of organisms. Under such conditions
it is difficult to definitively ascribe specific phenomenon to particular organisms.
In addition, Pfiesteria has not triggered any major fish kills or human
illness since 1998.
Anderson D (1994) Red tides. Sci. Am. (August), pp. 62-68.
Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA (2003). Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am. J. Trop. Med. Hyg. 68:65–69.
Burkholder JM (1999) The lurking perils of Pfiesteria. Sci. Am. (August), pp. 42-49.
Burkholder JM, Glasgow HB Jr (1997) Trophic controls on stage transformations of a toxic ambush-predator dinoflagellate. J. Euk. Microbiol. 44:200-205.
Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS (2003) Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin. Infect. Dis. 37:1304-1312.
Denney CF, Iragui VJ, Uber-Zak LD, Karpinski NC, Ziegler EJ, Visvesvara GS, Reed SL (1997) Amebic meningoencephlitis caused by Balamuthia mandrillaris: case report and review. Clin. Infect. Dis. 25:1354-1358.
Dunnebacke TH, Schuster FL, Yagi S, Booton GC (2004). Balamuthia mandrillaris from soil samples. Microbiology 150: 2837-2842.
Gelman BB, Rauf SJ, Nader R, Popov V, Borkowski J, Chaljub G, Nauta HW, Visvesvara GS (2001) Amoebic enceaphalitis due to Sappinia diploidea. JAMA 285:2450-2451.
Glasgow HB Jr, Burkholder JM, Schmechel DE, Tester PA, Rublee PA (1995) Insidious effects of a toxic estuarine dinoflagellated on fish survival and human health. J. Toxicol. Environ. Health 46: 501-522.
Jung S, Schelper RL, Visvesvara GS, Chang HT (2004) Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch. Pathol. Lab. Med. 128: 466-468.
Kaiser, J (2002) The science of Pfiesteria: elusive, subtle and toxic. Science 298: 346-349.
Lewis, RJ (2006) Ciguatera: Australian perspectives on a global problem. Toxicon 48: 799-809.
Martinez AJ, Visvesvara GS (1997) Free-living, amphizoic and opportunisitic amebas. Brain Path. 7:583-598.
Miller, T.R. and Belas, R. (2003) Pfiesteria piscicida, P. shumwayae, and other Pfiesteria-like dinoflagellates. Research in Microbiology 154: 85-90.
Recavarren-Arce S, Velarde C, Gotuzzo E, Cabrera J (1999) Ameoba angeitic lesions of the central nervous system in Balamuthia mandrilaris amoebiasis. Human Path. 30:269-273.
These pages are developed and maintained by Mark F. Wiser, Tulane University (©2000). Last update on May 22, 2018 .