| Lumen-Dwelling Protozoa |
|---|
|
Numerous protozoa inhabit the gastro-intestinal tract of humans (see Box). This list includes representatives from many diverse protozoan groups. The majority of these protozoa are non-pathogenic commensals, or only result in mild disease. Some of these organisms can cause severe disease under certain circumstances. For example, Giardia lamblia can cause severe acute diarrhea which may lead to a chronic diarrhea and nutritional disorders; Entamoeba histolytica can become a highly virulent and invasive organism that causes a potentially lethal systemic disease. Apicomplexa and microsporidia species (discussed elsewhere), which normally do not evoke severe disease, can cause severe and life-threatening diarrhea in AIDS patients and other immunocompromised individuals. Trichomonas vaginalis does not reside within the gastro-intestinal tract, but is often discussed with the intestinal flagellates. It infects the urogenital tract and and causes a sexually-transmitted disease.

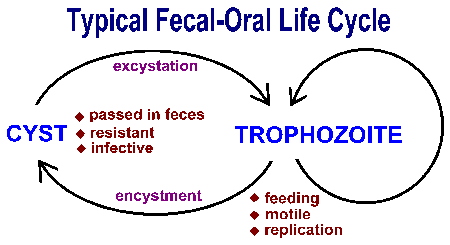
Intestinal protozoa are transmitted by the fecal-oral route and tend to exhibit similar life cycles consisting of a cyst stage and a trophozoite stage (Figure). Fecal-oral transmission involves the ingestion of food or water contaminated with cysts. After ingestion by an appropriate host, the cysts transform into trophozoites which exhibit an active metabolism and are usually motile. The parasite takes up nutrients and undergoes asexual replication during the trophic phase. Some of the trophozoites will develop into cysts instead of undergoing replication. Cysts are characterized by a resistant wall and are excreted with the feces. The cyst wall functions to protect the organism from desiccation in the external environment as the parasite undergoes a relatively dormant period waiting to be ingested by the next host. Factors which increase the likelyhood of ingesting material contaminated with fecal material play a role in the transmission of this intestinal protozoa (see Box). In general, situations involving close human-human contact and unhygenic conditions promote transmission.
poor personal hygiene
|
Giardia lamblia (also known as G. duodenalis, see comments on taxonomy) is a protozoan parasite that colonizes the upper portions of the small intestine. It has a worldwide distribution and is the most common protozoan isolated from human stools. The incidence is estimated at 200 million clinical cases per year. In fact, it was probably the first symbiotic protozoan ever observed. It is quite likely that Van Leeuwenhoek, the inventor of the microscope, first described Giardia in 1681in his own stools based upon his description of its characteristic movement. However, van Leeuwenhoek never submitted drawings of the organisms and Lambl is usually given credit for the identification of Giardia in the stools of pediatric patients in Praque in 1859.
Typically Giardia is non-invasive and often results in asymptomatic infections. Symptomatic giardiasis is characterized by acute or chronic diarrhea and/or other gastro-intestinal manifestations.
Giardia exhibits a typical fecal-oral transmission cycle (see above). The infection is acquired through the ingestion of cysts. Factors leading to contamination of food or water with fecal material are correlated with transmission (Box). For example, giardiasis is especially prevalent in children and particularly those children in institutions or day-care centers. In developing countries, poor sanitation contributes to the higher levels of giardiasis, and water-borne outbreaks due to inadequate water treatment have also been documented. Backpackers in areas of no human habitation are believed to acquire from drinking from streams and some data suggest that beavers are the reservoir. However, the zoonotic transmission of Giardia is controversial and has not been unambiguously demonstrated. It is not clear whether Giardia lamblia represents a single species capable of infecting a wide range of animals, or whether each host has their own 'pet' Giardia. Evidence indicating that Giardia transmission between dogs and humans is quite rare favors the latter. Molecular evidence suggests that some isolates exhibit narrow host ranges whereas others exhibit wide host ranges (see notes on taxonomy). Regardless of whether zoonotic transmission is possible, person-to-person transmission is the most prevalent mode of transmission and the risk factors are close human contact combined with unhygienic conditions.
The ingested cyst passes through the stomach and excystation takes place in the duodenum. Excystation can be induced in vitro by a brief exposure of the cysts to acidic pH (~2) or other sources of hydrogen ions. This exposure to the acidic pH mimics the conditions of the stomach and probably functions as an environmental cue for the parasite. Flagellar activity begins within 5-10 minutes following the acid treatment and the trophozoite emerges through a break in the cyst wall. The breakdown of the cyst wall is believed to be mediated by proteases. The trophozoite will undergo cytokinesis (cell division without nuclear replication) within 30 minutes after emerging from the cyst resulting in two binucleated trophozoites.
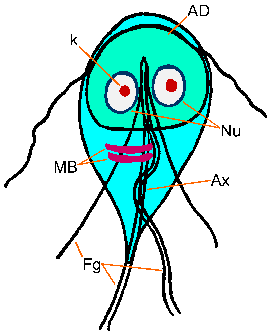 The
Giardia trophozoite exhibits a characteristic pear, or tear-drop,
shape with bilateral symmetry when viewed from the top (Figure). It is typically
12-15 Ám long, 5-10 Ám wide, and 2-4 Ám thick. Characteristic features of the
stained trophozoite include: two nuclei (Nu) with central karyosomes (k), fibrils
running the length of the parasite, and median bodies (MB). The large karyosome
and lack of peripheral chromatin gives the nuclei a halo appearance. The fibrils
are called axonemes (Ax) and are formed from the proximal regions of the flagella
(Fg) within the body of the trophozoite. The median bodies are a pair of curved
rod-shaped structures which lie posterior to the nuclei. At the ultrastructural
level the median bodies contain an array of microtubules. The function of the
median bodies is not known, but most believe they are somehow involved with
the adhesive disk and its formation. An adhesive disk (AD), not always visible
by light microscopy, occupies the ventral side of the anterior end.
The
Giardia trophozoite exhibits a characteristic pear, or tear-drop,
shape with bilateral symmetry when viewed from the top (Figure). It is typically
12-15 Ám long, 5-10 Ám wide, and 2-4 Ám thick. Characteristic features of the
stained trophozoite include: two nuclei (Nu) with central karyosomes (k), fibrils
running the length of the parasite, and median bodies (MB). The large karyosome
and lack of peripheral chromatin gives the nuclei a halo appearance. The fibrils
are called axonemes (Ax) and are formed from the proximal regions of the flagella
(Fg) within the body of the trophozoite. The median bodies are a pair of curved
rod-shaped structures which lie posterior to the nuclei. At the ultrastructural
level the median bodies contain an array of microtubules. The function of the
median bodies is not known, but most believe they are somehow involved with
the adhesive disk and its formation. An adhesive disk (AD), not always visible
by light microscopy, occupies the ventral side of the anterior end.
Giardia trophozoites possess four pairs of flagella and are motile. Three pairs of flagella emerge from the dorsal surface (anterior, posterior-lateral, caudal) and one pair emerges from the ventral surface. Trophozoites exhibit a distinctive erratic twisting motion, sometimes compared to that of a falling leaf. However, the trophozoites are predominantly found attached to epithelial cells of the small intestine (especially the duodenum and jejunum) and are rarely found in stools, except in the cases of severe diarrhea. This attachment to the intestinal epithelium is mediated by an organelle on the ventral side of the parasite referred to as the adhesive disk (see below). The trophozoite absorbs nutrients from the intestinal lumen via pinocytosis and no specialized feeding organelles have been described.
The trophic stage is also characterized by an asexual replication. Both nuclei divide at about the same time and cytokinesis restores the binucleated state. Each daughter cell receives one copy of each nuclei. Both nuclei appear equal in regards to gene expression and other properties.
 As
an alternative to replication the trophozoite can encyst. During encystment
the parasite rounds up, detaches from the intestinal epithelium, and secretes
a cyst wall. Encystation can also be carried out in vitro. Optimal induction
of encystment is obtained by depriving the trophozoites of bile at pH 7 followed
by an exposure to high concentrations of bile at pH 7.8. The lack of bile at
neutral pH mimics the conditions under the mucus blanket adjacent to the intestinal
epithelial cells, whereas exposure to high concentrations of bile at more alkaline
pH is analogous to the intestinal lumen. These studies highlight the extent
to which Giardia has adapted to life within the gastrointestinal tract.
As
an alternative to replication the trophozoite can encyst. During encystment
the parasite rounds up, detaches from the intestinal epithelium, and secretes
a cyst wall. Encystation can also be carried out in vitro. Optimal induction
of encystment is obtained by depriving the trophozoites of bile at pH 7 followed
by an exposure to high concentrations of bile at pH 7.8. The lack of bile at
neutral pH mimics the conditions under the mucus blanket adjacent to the intestinal
epithelial cells, whereas exposure to high concentrations of bile at more alkaline
pH is analogous to the intestinal lumen. These studies highlight the extent
to which Giardia has adapted to life within the gastrointestinal tract.
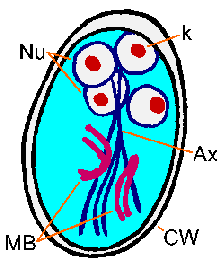
Molecular and ultrastructural studies reveal the synthesis of cyst wall proteins and the appearance of large secretory vesicles in the parasite cytoplasm follow the induction of encystment. After cyst wall formation the parasite undergoes one round of nuclear division without cytokinesis resulting in four nuclei. These four nuclei (Nu) are usually located at the anterior end of the cyst (Figure). The flagella and adhesive disk are lost as the cyst matures, but the axonemes (Ax) and median bodies (MB) persist. The distinctive fibrils (ie, axonemes), which extend across the length of the cyst, result in Giardia being relatively easy to unambiguously identify. The cysts are oval shaped and typical measure 11-14 µm in length and 6-10 µm wide. Other characteristics of Giardia cysts include a well-defined wall (CW) which is often set apart from the cytoplasm of the parasite. The cysts are passed in the feces and can survive for up to three months under appropriate temperature and moisture conditions. Mature cysts are infective to the next host that happens to ingest them, thus completing the life cycle.
 |
 |
 |
 |
A unique ultrastructural feature of Giardia is the adhesive disk (also called ventral disk, sucking disk, sucker, or striated disk). The adhesive disk is a concave structure which occupies approximately two-thirds of the anterior end of the ventral surface (Figure, left panel). As the names imply, this structure plays a role in the attachment of the trophozoite to the intestinal epithelium and ultrastructural studies reveal close associations between the adhesive disk and the intestinal brush border (Figure, upper right panel). (Click here for larger image.)
The
adhesive disk appears to be a relatively rigid structure and striations are
evident by transmission electron microscopy. These striations are the result
of microtubules (mT) and a unique cytoskeletal element called microribbons (mR).
Microribbons are long flattened structures and each microribbon is associated
with a microtubule (Figure, middle right panel). The combined microtubule-microribbon
structure are arranged in concentric rows that form a flatten spiral with minimal
overlap. The outer rim of the adhesive disk, called the lateral crest, contains
components of the actin-myosin cytoskeleton.
This association of proteins involved in the generation of contractile force and other cytoskeletal elements in the adhesive disk suggests that attachment is mediated by mechanical forces generated by the parasite. The observation that imprints and circular dome-shaped lesions remain in the intestinal brush border (ie, microvilli) following detachment of trophozoites (Figure, lower right panel) is consistent with contractile forces playing a role in attachment. Other proposed mechanisms for the attachment of Giardia to the intestinal epithelium include hydrodynamic forces generated by the ventral flagella and receptor-mediated binding via lectins on the trophozoite surface. However, flagellar movement is poorly correlated with attachment and the surface lectins cover the entire trophozoite and are not specifically localized to the adhesive disk.
 |
 |
 |
 |
The clinical features associated with Giardia infection range from total latency (ie, asymptomatic), to acute self-resolving diarrhea, to chronic syndromes associated with nutritional disorders, weight loss and failure to thrive. Children exhibit clinical symptoms more frequently that adults and subsequent infections tend to be less severe than initial infections. The incubation period is generally 1-2 weeks, but ranges of 1-75 days have been reported.
The first signs of acute giardiasis include nausea, loss of appetite and an upper gastro-intestinal uneasiness. These signs are often followed or accompanied by a sudden onset of explosive, watery, foul-smelling diarrhea. Stools associated with Giardia infection are generally described as loose, bulky, frothy and/or greasy with the absence of blood or mucus, which may help distinguish giardiasis from other acute diarrheas. Other gastro-intestinal disturbances associated with giardiasis include: flatulence, bloating, anorexia, cramps, and foul sulfuric belching (sometimes called 'purple burbs'). The acute stage usually resolves spontaneously in 3-4 days and is often not recognized as being giardiasis. Occasionally, though, an acute infection will persist and lead to malabsorption, steatorrhea (excessive loss of fat in the feces), debility (loss of strength) and weight loss. Some of the individuals who resolve the acute symptoms do not clear the infection, but become asymptomatic cyst passers without clinical manifestations, whereas others may have a few sporadic recurrences of the acute symptoms.
Acute infections can also develop into long-standing subacute or chronic infections which in rare cases last for years. The typical chronic stage patient presents with recurrent brief episodes of loose foul stools which may be yellowish, frothy and float, accompanied by intestinal gurgling, abdominal distention and flatulence. Between episodes the stools are usually mushy, but normal stools or constipation can also occur. Cramps are uncommon during chronic infections, but sulfuric belching is frequent. Anorexia, nausea, and epigastric uneasiness are additional frequent complaints during chronic infections. In the majority of chronic cases the parasites and symptoms spontaneously disappear.
| Click for larger image |
The specific mechanisms of Giardia pathogenesis leading to diarrhea and intestinal malabsorption are not completely understood and no specific virulence factors have been identified. Attachment of trophozoites to the brush border could produce a mechanical irritation or mucosal injury. In addition, normal villus structure is affected in some patients. For example, villus blunting (atrophy) and crypt cell hypertrophy and an increase in crypt depth have been observed to varying degrees. The increase in crypt cells will lead to a repopulation of the intestinal epithelium by relatively immature enterocytes with reduced absorptive capacities. An increased inflammatory cell infiltration in the lamina propria has also been observed and this inflammation may be associated with the pathology. Giardia infection can also lead to lactase deficiency (see lactose intolerance below) as well as other enzyme deficiencies in the microvilli. This reduced digestion and absorption of solutes may lead to an osmotic diarrhea and could also explain the malabsorption syndromes. Thus far, no single virulence factor or unifying mechanism explains the pathogenesis of giardiasis. [See also Pathophysiology of Diarrhea for a general discussion of diarrhea.]
Post-Giardia Lactose Intolerance. Some patients may present with a lactose intolerence during active Giardia infections which can persist after parasite clearance. This clinical manifestation is due to the parasite-induced lactase deficiency and is most common in ethnic groups with a predisposition for lactase deficiency. Lactase is an enzyme that breaks down lactose, a sugar found in milk, to monosaccharides which can be absorbed. This lactose intolerence syndrome should be considered in persons who still present mushy stools and excessive gas following treatment, but have no detectable parasites.
 |
 |
 |
 |
|
Parasite Detection |
Stool Examination
Duodenal Aspirate or Biopsy
|
Diagnosis is confirmed by finding cysts or trophozoites in feces or in duodenojejunal aspirates or biopsies. Detection of the parasites can be difficult since Giardia does not appear consistently in the stools of all patients. Some patients will express high levels of cysts in nearly all the stools, whereas others will only exhibit low parasite counts in some of the stools. A mixed pattern, in which periods of high cyst excretion alternate with periods of low excretion, has also been observed. In addition, parasites are easier to find during acute infections than chronic infections. Aspiration and biopsy may also fail to confirm the infection due to patchy loci of infection, and some question the usefulness of these invasive procedures.
Stool examination is the preferred method for Giardia diagnosis. Three stools taken at intervals of at least two days should be examined. Watery or loose stools may contain motile trophozoites which are detectable by the immediate examination of wet smears. Otherwise the specimen should be preserved and stained due to trophozoite lability. The hardier cysts are relatively easy to recognize in either direct or stained smears (see cyst morphology). In addition, diagnostic kits based on immunofluorescence or the detection of copro-antigens are also available.
Diagnosis can also be made by examining duodenal fluid for trophozoites. Duodenal fluid is obtained by either intubation or the Enterotest® (also called 'string test'). The Enterotest® consists of a gelatin capsule containing a nylon string of the appropriate length. The free end of the string is taped to the patient's face and the capsule is swallowed. After four hours to overnight the string is retrieved and the bile-stained mucus on the distal portion of the string is scraped off and examined by both wet mount and permanent staining. A small intestinal biopsy, preferably from multiple duodenal and jejunal sites, may also reveal trophozoites attached to the intestinal epithelium. [The small intestine is divided into 3 sections: the duodenum (first or proximal portion after the stomach); the jejunum (the middle portion); and the ileum (the distal or last portion before the large intestine).]
 |
 |
 |
 |
Infected individuals should be treated since Giardia can persist and lead to severe malabsorption syndromes and weight loss. Treatment is effective at reducing morbidity and there are no sequelae. Metronidazole (Flagyl®), although not licensed in the United States for giardiasis, effectively clears the parasite (cure rates approximately 85%) and is the drug of choice. The recommended dosage is 750 mg three times per day for five days (or at least >3 days). For children 15 mg/kg/d in three doses is recommended. Other effective drugs include: quinacrine (Atabrine®), tinidazole (Fasigyn®), furazolidone (Furoxone®), and paramomycin (Humatin®). Tinidazole is effective as a single two gram dose; paramomycin is not absorbed and may be useful during pregnancy.
The widespread distribution of Giardia and the infectivity of the cysts make it unlikely that human infection will be completely eliminated. Control measures to prevent or reduce Giardia infection will depend on the specific circumstances of transmission, but in general involve measures which prevent the ingestion of substances contaminated with fecal material (see fecal-oral transmission factors). Health promotion and education aimed at improving personal hygiene, and emphasizing hand washing, sanitation and food handling, are effective control activities for the reduction of person-to-person transmission. Special attention to personal hygiene in high-risk situations such as day-care centers and other institutions is needed. Treatment of asymptomatic household members prevents reinfection in non-endemic areas. However, the value of treating asymptomatic carriers in hyperendemic communities is questionable since reinfection rates are high. The socio-economic situation in many developing countries makes it difficult to prevent infection. Public health measures to protect water supplies from contamination are required to prevent epidemics and to reduce endemicity. Tourists should not drink tap water without additional treatment in places where purity is questionable. Boiling or iodine treatment kills Giardia cysts, but standard chlorination does not. There are no safe or effective chemoprophylatic drugs for giardiasis.
 |
 |
 |
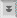 |
The trichomonads are a group of flagellated protozoa. Most of the members of this group are parasitic and only a few free-living species have been identified. Generally the trichomonads are non-pathogenic commensals and only a few species are of importance in animals and humans. Four species of trichomonads infect humans (Table). Among these only Trichomonas vaginalis is clearly pathogenic and it is usually of low virulence. The others exhibit a questionable pathogenicity.
| Trichomonads of Humans |
||||||||||
|
The trichomonads of humans inhabit different anatomical locations. T. vaginalis is a common sexually transmitted disease found in the uro-genital tract. T. tenax, also called T. buccalis, is a commensal of the human oral cavity, found particularly in patients with poor oral hygiene and advanced periodontal disease. T. tenax, or an organism with similar morphology is also occasionally found in the lungs. Such cases have reported mainly in patients with underlying cancers or other lung diseases or following surgery. Pentatrichomonas hominis, formerly known as Trichomonas hominis, is a non-pathogenic commensal of the large intestine (see non-pathogenic intestinal flagellates). Some authors divide the trichomonads into three genera based on the number of free flagella. Species with three flagella are called Tritrichomonas, those with four are called Trichomonas, and Pentatrichomonas refers to trichomonads with five free anterior flagella. Dientamoeba fragilis was originally believed to be an ameba (see non-pathogenic intestinal ameba). Now it is know to be a flagellate—however without flagella—related to the trichomonads.
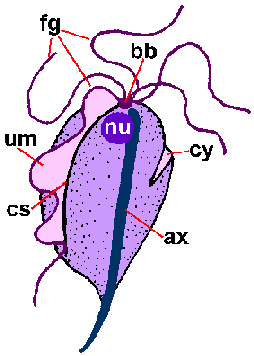
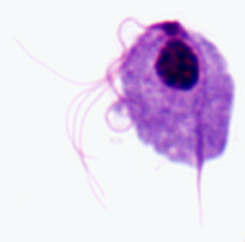
A distinctive feature of the trichomonads is an axostyle (ax) which runs the length of the organism and appears to protrude from the posterior end (Figure). The axostyle is a cytoskeletal element composed of concentric rows of microtubules and is believed to function in the attachment of the parasite to epithelial cells. Trichomonads are also characterized by 4-6 flagella (fg) emerging from the anterior end. One of the flagella is attached to the body of the organism and forms a posteriorly-directed undulating membrane (um), whereas the remaining flagella are free. The combined basal bodies (bb) and the base of the undulating membrane, called the costa (cs), are often seen is stained preparations. Less frequently seen is the cytostomal groove (cy). A single nucleus (nu) is found at the anterior end of the parasite.
 |
 |
 |
| Schematic representation of major structural features of trichmonads (left). Giemsa-stained trophozoite of T. vaginalis from in vitro culture (middle). Electron micrograph of axostyle cross-section showing concentric rows of microtubules (right). | ||
The trichomonads, like many other intestinal protozoa, exhibit an anerobic
metabolism and lack mitochondria. Part of energy metabolism of trichomonads
involves a unique organelle called the hydrogenosome. The hydrogenosome has
a double membrane and is distantly related to the mitochondrion. However, it
lacks DNA, cytochromes and many typical mitochnondrial functions such as enzymes
of the tricarboxylic acid cycle and oxidative phosphorylation. The primary function
of the hydrogenosome is the metabolism of pyruvate, produced during glycolysis
within the cytosol, to acetate and carbon dioxide with the concomitant production
of ATP. The electrons release from the oxidation of pyruvate are transferred
to hydrogen ions to produce molecular hydrogen, hence the name hydrogenosome.
 |
 |
 |
 |
Trichomonas vaginalis was first described from purulent vaginal discharges in 1836 and by the early part of the twentieth century was recognized as an etiological agent of vaginitis. Trichomoniasis is a common sexually transmitted disease with a worldwide distribution and an estimated 167 million people becoming infected per year worldwide and 5 million new infections per year in the United States. Trichomoniasis is believed to be the most common non-viral sexually transmitted disease. Despite the frequency of trichomoniasis it has in the past been considered more of a nuisance parasite rather than a major pathogen. However it is now recognized a factor in promoting HIV infection (see Box), causing low-weight and premature births, and predisposing women to substantial discomfort and stress.
| Trichomonas and HIV |
The pathology
caused by Trichomonas may enhance the efficiency of HIV transmission
(1). T. vaginalis infection typically elicits a local cellular
immune response with inflammation of the vaginal epithelium and cervix in
women and the urethra of men. This inflammatory response includes the infiltration
of potential HIV target cells such as CD4+ bearing lymphocytes and macrophages.
In addition, T. vaginalis can cause punctate hemorrhages on the
vaginal walls and cervix. This leukocyte infiltration and the genital lesions
may increase the number of target cells for the virus and allowing direct
viral access to the bloodstream through open lesions. In addition, the hemorrhages
and inflammation can increase the level of virus in body fluids and the
numbers of HIV-infected lymphocytes and macrophages present in the genital
area in persons already infected with HIV. This increase of free virus and
virus-infected leukocytes can increase the probability of HIV exposure and
transmission to an uninfected partner. Increased cervical shedding of HIV
has been shown to be associated with cervical inflammation, and substantially
increased viral loads in semen have been documented in men with trichomoniasis.
Moreover, since many patients with Trichomonas infection are asymptomatic,
or only mildly symptomatic, they are likely to remain sexually active in
spite of infection.
|
T. vaginalis, despite its name, infects both men and women. In females the organism primarily inhabits the vagina, and in males it is usually found in the urethra, prostate or epididymis. The life cycle consists only of a trophozoite stage which is transmitted by direct contact during sexual intercourse. Non-venereal transmission is rare, but possible since the trophozoites can survive 1-2 days in urine and 2-3 hours on a wet sponge. In addition, neonatals have been infected during the birth process. The trophozoites live closely associated or attached to the epithelium of the urogenital tract, where they replicate by binary fission.
 |
 |
 |
 |
| Clinical Manifestations |
|
| Females |
Males |
|
|
| *% of infected; **% of symptomatic | |
T. vaginalis causes different clinical manifestations in men and women and women (Table) are more likely to exhibit symptoms which tend to persist longer. The incubation period typically ranges from 4-28 days. In females the infection can present as a mild vaginitis, an acute or chronic vulvovaginitis, or urethritis. The onset or exacerbation of symptoms commonly occurs during or immediately after menstration. The most common complaint associated with T. vaginalis infection is a persistent mild vaginitis associated with a copious, foul-smelling discharge that is often accompanied by burning or itching. This discharge is most often gray, but can be yellow or green and is occasionally frothy or blood tinged. The discharge diminishes as the infection becomes more chronic. Many women also experience painful or difficult coitus. Urethral involvement occurs in a large number of cases and is characterized by dysuria (painful urination) and frequent urination.
The vaginal epithelium is the primary site of infection. Thus the vaginal walls are usually erythematous (i.e., red) and may show petechial (a small non-raised spot) hemorrhages. Punctate hemorrhages of the cervix, called strawberry cervix, are observed in approximately 2% of the cases. This strawberry cervix is a distinctive pathological observation associated with trichomonasis not seen with other sexually transmitted diseases.
Males are likely to be asymptomatic (50-90%) and the infection tends to be self-limiting. The urethra and prostate are the most common sites of infection. Common symptoms include: urethral discharge (ranging from scant to purulent), dysuria, and urethral pruritus (itching). Some men experience burning immediately after coitus.
Little is known about the pathophysiology associated with T. vaginalis infection, but is presumably due to interactions between the parasite and host epithelial cells. In vitro studies indicate that T. vaginalis can destroy cells in a contact dependent manner. Therefore adhesion of the trophozoites to the epithelium is believed to be a major factor in the pathogenesis. Several adhesion proteins have been identified on the surface of the trophozoites. In addition, secreted proteases that could play a role in pathogenesis have also been identified.
 |
 |
 |
 |
In general, the clinical manifestations are not reliable as sole means of diagnosis since the clinical presentation is similar to other STDs and many patients have mild or no symptoms. Diagnosis is confirmed by the demonstration of trophozoites in vaginal, urethral, prostatic secretions, or urine sediment (following prostate massage). Microscopic examination of wet mounts of fresh vaginal discharge, preferably collected with a speculum on a cotton-tipped applicator, is the most practical method of diagnosis. Specimens should be diluted in saline and examined immediately. T. vaginalis is recognized by its characteristic morphological features (see above) and its rapid jerky motility. Specimens can also be fixed and stained with Giemsa or fluorescent dyes. However, the organism may be difficult to recognize on stained slides.
The sensitivity of direct observation ranges from 40-80%. Therefore, in vitro culture is considered the gold standard for diagnosis despite some limitations. For example, access to facilities is needed and organisms require 2-7 days of growth before they are detected. The accessibility issue is partly resolved by the InPouch™TV culture system (Biomed Diagnostics). This is a commercially available self-contained system for the detection of T. vaginalis in clinical specimens. Antibody and DNA-based tests with high sensitivity and specificity are being developed.
Metronidazole (Flagyl®) and other nitroimidazoles, such as tinidazole, are highly effective against trichomoniasis. The metronidazole is activated by the hydrogensome to a nitro radical ion intermediate. Either a single two gram dose (85-92% cure rate) or 250 mg three time daily for 7-10 days (>95% cure rate) can be used. Sexual partners should be treated at the same time to prevent reinfection. Some drug resistance has been reported, but this is not a wide-spread problem. Treatment failures are generally due to noncompliance or reinfection.
| Trichomoniasis as an STD |
|
Reviews on Trichomoniasis:
 |
 |
 |
 |
Dientamoeba fragilis was originally described as an ameba based upon its morphology. However, later it was recognized to exhibit a morphology more similar to the turkey parasite Histomonas meleagridis, except for the lack of flagella. Ultrastructural studies also suggest similarities to the trichomonads, including the possession of hydrogenosomes and molecular studies have confirmed a close phylogenetic relationship between Dientamoeba and Histomonas and a possible more distal relationship to Trichomonas.
As with other trichomonads, Dientamoeba only exhibits a trophozoite stage (Figure). This raises some questions about the mode of transmission in that a cyst stage is usually involved in fecal oral transmission. In addition, the trophozoites of Dientamoeba survive outside of the body for a very short time. H. meleagridis also lacks a cyst stage and has been demonstrated to be transmitted via the eggs of a nematode. Due to the close relationship between Histomonas and Dientamoeba, it is proposed that Dientamoeba is also transmitted via helminth eggs. Epidemiological and experimental evidence tends to incriminate the pinworm Enterobius vermicularis as the carrier for Dientamoeba. More recently, pigs have been shown to be a natural host for D. fragilis of the same genotype as found in humans, thus raising the possibility of a zoonotic transmission (Cacciò et al, Emerg Infect Dis 18(5):838–841, 2012).
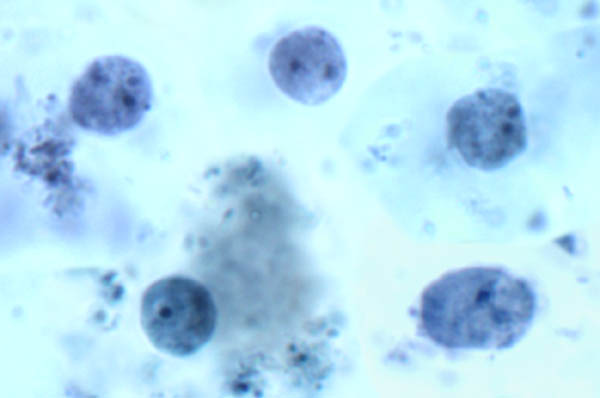 |
| Morphology of Dientamoeba fragilis from a stool sample. Trophozoites exhibit an ameba-like morphology and are often bi-nucleated. |
Historically Dientamoeba has been considered as a non-pathogenic commensal. However, clinical symptoms often correlate with the presence of large numbers of trophozoites and treatment of the infection resolves the symptoms. The incidence of symptoms is estimated at 15-30% of infected individuals. Clinical symptoms associated with Dientamoeba include intermittent diarrhea, abdominal pain, flatulence, nausea and fatigue. Little is known about the pathogenesis and Dientamoeba probably acts as a low-grade irritant of intestinal mucosal surfaces that may lead to some inflammation. Iodoquinol is generally the drug of choice for the treatment of Dientamoeba. Paromomycin and metronidazole are also effective.
Reviews on Dientamoeba
 |
 |
 |
 |
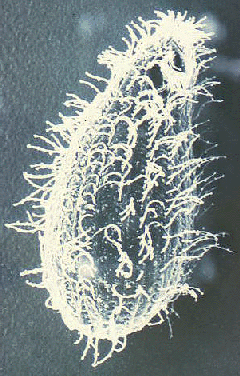 Balantidium
coli is the only ciliate which infects humans. It is found world wide, but
like many other fecal-oral transmitted diseases, it is more prevalent in the
tropics. However, prevalence rates rarely exceed 1%. B. coli also infects
a wide variety of mammals and is especially common in monkeys and pigs. Prevalence
in pigs ranges from 20–100% and human balantidiosis usually exhibits an
increased prevalence in communities that live in close association with pigs.
For example, in Papua New Guinea, where pigs are the principal domestic animals,
the prevalence among swine herders and slaughterhouse workers has been reported
to be as high as 28%. Human-to-human transmission has also been documented and
this mode of transmission is likely to occur in environments with crowding and
poor personal hygiene such as mental hospitals and prisons. (Skip
general ciliate biology)
Balantidium
coli is the only ciliate which infects humans. It is found world wide, but
like many other fecal-oral transmitted diseases, it is more prevalent in the
tropics. However, prevalence rates rarely exceed 1%. B. coli also infects
a wide variety of mammals and is especially common in monkeys and pigs. Prevalence
in pigs ranges from 20–100% and human balantidiosis usually exhibits an
increased prevalence in communities that live in close association with pigs.
For example, in Papua New Guinea, where pigs are the principal domestic animals,
the prevalence among swine herders and slaughterhouse workers has been reported
to be as high as 28%. Human-to-human transmission has also been documented and
this mode of transmission is likely to occur in environments with crowding and
poor personal hygiene such as mental hospitals and prisons. (Skip
general ciliate biology)
Ciliates are a large and diverse group of protozoa. Most ciliates are free-living and are found in a variety of habitats. Well-known ciliates include Paramecium species, which are found in ponds throughout the world, and Ichthyophthirius multifiliis, an ectoparasite of fish that causes white spot disease (also called 'ick'). As the name implies, ciliates possess cilia at some point during their life cycles. The cilia are generally arranged in longitudinal rows and typically cover the surface of the organism. Ciliates are also characterized by nuclear dimorphism in that they have two distinct nuclei. The large kidney-shaped macronucleus is involved in the 'housekeeping' or somatic functions of the cell, whereas the smaller spherical micronucleus contains the complete genome. The macronucleus contains thousands of copies of transcriptionally active 'minichromosomes' representing 10-20,000 different DNA molecules. This large number of telomeres (chromosome ends) resulted in ciliates being an early model system for the study of telomeres and telomerase (the enzyme that synthesizes telomeres).
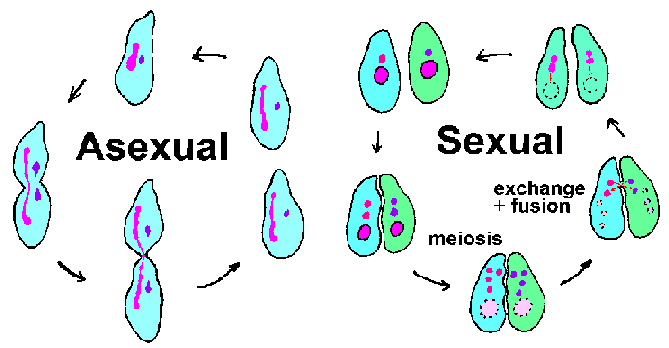
Ciliates undergo both an asexual reproduction (ie, binary fission) and a sexual reproduction involving conjugation (Figure above). During conjugation, two ciliates of opposite mating types pair and exchange genetic material. Conjugal contact triggers meiosis in the micronuclei resulting in 4 haploid micronuclei. Concurrently, the macronucleus breaks down and disappears. Three of the micronuclei disintegrate and the remaining micronucleus divides again. Each of the conjugating organisms donates a micronucleus (gametic or male) to its mate via a cytoplasmic bridge that connects them. The gametic micronucleus fuses with the stationary (or female) micronucleus forming the diploid zygotic micronucleus. The conjucating pair separates and the zygotic nucluei undergo another round of division. One of these micronuclei develops into the the macronucleus, thus completing the cycle. Formation of the macronucleus involves fragmentation of the chromosomes and loss of DNA sequences corresponding to genes not expressed at high levels during the normal asexual cycle. The remaining DNA fragments, or minichromosomes, are then amplified. (See diagram of DNA processing during macronucleus formation.)
 BALANTIDOSIS
BALANTIDOSISB. coli usually lives as a non-pathogenic commensal in the large intestine and produces no symptoms. Superficial inflammation of the colonic mucosa may occur which can result in diarrhea and colicky pain. Mild or chronic infections are characterized by intermittent diarrhea and constipation, weight loss, and abdominal pain. On rare occasions the trophozoites will invade the intestinal epithelium and produce ulceration. Clinically this results in an acute diarrhea with mucus and blood (ie, dysentery). This balantidial dysentery is similar to the dysentery produced by Entameoba histolytica (see below). Rare extra-intestinal infections involving lungs, vagina, ureter and urinary bladder and intestinal perforations leading to peritonitis have been reported.
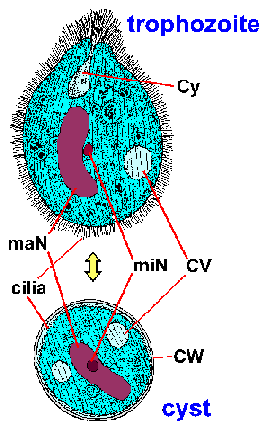
Laboratory diagnosis is made by identifying the organism in feces. Balantidium exhibits a typical fecal-oral life cycle consisting of trophozoite and cyst stages. The large size and unique morphological features of Balantidium (Figure) precludes its confusion with any other protozoa found in human feces. The trophozoite is ovoid and has an average size of 70 x 45 µm, but can range upwards to 150-200 Ám. The cyst has a distinctive cyst wall (CW) and is more spherical with an average diameter of 55 Ám. In stained specimens the most obvious internal structure is the large macronucleus (maN). The micronucleus (miN) may not always be apparent because of its close association with the macronucleus. Contractile vacuoles (CV), which function in osmotic regulation, are often visible and occasionally the cytostome (Cy) is detectable. Similar to many other ciliates, Balantidium is covered by rows of cilia. The cilia give the parasite surface a fuzzy appearance and are less pronounced in the cyst stage.
The treatment of choice is tetracycline given at 500 mg four times per day for 10 days. Iodoquinol is the recommended alternate drug. Metronidazole has not produced consistent results. Preventive measures are the same as other diseases transmitted by the fecal-oral route (see fecal-oral transmission factors or discussion of Giardia prevention). In addition, pig sewerage should be kept away from supplies of drinking water and food.
 |
 |
 |
 |
Several members of the genus Entamoeba infect humans (see below). Among these only E. histolytica is considered pathogenic and the disease it causes is called amebiasis or amebic dysentery. E. dispar is morphologically identical to E. histolytica and the two were previously considered to be the same species. However, genetic and biochemical data indicate that the non-pathogenic E. histolytica is a distinct species (see discussion of criteria). The two species are found throughout the world, but like many other intestinal protozoa, they are more common in tropical countries or other areas with poor sanitary conditions. It is estimated that up to 10% of the world's population may be infected with either E. histolytica or E. dispar and in many tropical countries the prevalence may approach 50%. There are an estimated 50 million cases of amebiasis per year and up to 100,000 deaths.
 |
 |
 |
 |
E. histolytica exhibits a typical fecal-oral life cycle consisting of infectious cysts passed in the feces and trophozoites which replicate within the large intestine. The infection is acquired through the ingestion of cysts and the risk factors are similar to other diseases transmitted by the fecal-oral route (see Table). Contaminated food and water are probably the primary sources of infection. The higher prevalence in areas of lower socioeconomic status is likely due to poor sanitation and a lack of indoor plumbing. However, E. histolytica is rarely the cause of travelers' diarrhea and is usually associated with a long-term (>1 month) stay in an endemic area. A higher prevalence of E. histolytica infection is also observed in institutions, such as mental hospitals, orphanages and prisons, where crowding and problems with fecal contamination are contributing factors. A high prevalence among male homosexuals has also been noted. Humans are the only host of E. histolytica and there are no animal reservoirs.
 Upon
ingestion the cysts pass through the stomach and excyst in the lower portion
of the small intestine. Excystation involves a disruption of the cyst wall and
the quadranucleated ameba emerges through the opening. The ameba undergoes another
round of nuclear division followed by three successive rounds of cytokinesis
(ie, cell division) to produce eight small uninucleated trophozoites, sometimes
called amebulae. These immature trophozoites colonize the large intestine, especially
the cecal and sigmoidorectal regions, where they feed on bacteria and cellular
debris and undergo repeated rounds of binary fission.
Upon
ingestion the cysts pass through the stomach and excyst in the lower portion
of the small intestine. Excystation involves a disruption of the cyst wall and
the quadranucleated ameba emerges through the opening. The ameba undergoes another
round of nuclear division followed by three successive rounds of cytokinesis
(ie, cell division) to produce eight small uninucleated trophozoites, sometimes
called amebulae. These immature trophozoites colonize the large intestine, especially
the cecal and sigmoidorectal regions, where they feed on bacteria and cellular
debris and undergo repeated rounds of binary fission.
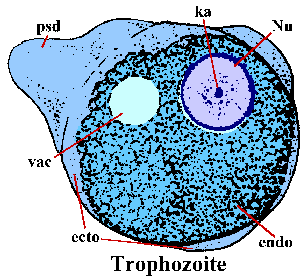
E. histolytica trophozoites have an amorphous shape and are generally 15-30 µm in diameter. The trophozoites move by extending a finger-like pseudopodium (psd) and pulling the rest of the body forward (called ameboid movement). The pseudopodia, and sometimes the outer edge of the trophozoite, have a clear refractile appearance and is referred to as the ectoplasm (ecto). The rest of the cytoplasm has a granular appearance and is called the endoplasm (endo). Occasionally a glycogen vacuole (vac) is evident. Nuclear (Nu) morphology in stained specimens is characterized by a finely granular ring of peripheral chromatin and a centrally located karyosome (ka).
 As
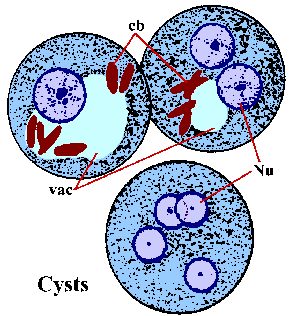
an alternative to asexual replication trophozoites can also encyst. The factors
responsible for the induction of encystation are not known. Encystation begins
with the trophozoites become more spherical and the appearance of chromatoid
bodies in the cytoplasm. Chromatoid bodies (cb) are stained elongated structures
with round ends and represent the aggregation of ribosomes. The cyst wall is
composed of chitin and has a smooth refractile appearance. Cyst maturation involves
two rounds of nuclear replication without cell division and cysts with 1-4 nuclei
(Nu) are found in feces. The nuclear morphology of the cyst is similar to that
of the trophozoite except that the nuclei become progressively smaller following
each division. Sometimes the young cysts (ie, 1-2 nuclei) will have a glycogen
vacuole (vac) which will appear as a clear area in stained specimens. This vacuole
will sometimes displace and alter the morphology of the nuclei. The chromatoid
bodies tend to disappear as the cyst matures. The cysts are generally 12-15
Ám in diameter. Cysts are immediately infective upon excretion with the feces
and will be viable for weeks-to-months depending on environmental conditions.
As
an alternative to asexual replication trophozoites can also encyst. The factors
responsible for the induction of encystation are not known. Encystation begins
with the trophozoites become more spherical and the appearance of chromatoid
bodies in the cytoplasm. Chromatoid bodies (cb) are stained elongated structures
with round ends and represent the aggregation of ribosomes. The cyst wall is
composed of chitin and has a smooth refractile appearance. Cyst maturation involves
two rounds of nuclear replication without cell division and cysts with 1-4 nuclei
(Nu) are found in feces. The nuclear morphology of the cyst is similar to that
of the trophozoite except that the nuclei become progressively smaller following
each division. Sometimes the young cysts (ie, 1-2 nuclei) will have a glycogen
vacuole (vac) which will appear as a clear area in stained specimens. This vacuole
will sometimes displace and alter the morphology of the nuclei. The chromatoid
bodies tend to disappear as the cyst matures. The cysts are generally 12-15
Ám in diameter. Cysts are immediately infective upon excretion with the feces
and will be viable for weeks-to-months depending on environmental conditions.
 |
 |
 |
 |
| Amebiasis Progression |
| non-invasive
invasive
|
E. histolytica frequently lives as a commensal within the large intestine with no overt clinical manifestations. However, trophozoites can invade the colonic epithelium and produce ulcers and dysentery (see Box). This invasive disease can become progressively worse and lead to a more serious disease. The amebas can also metastasize to other organs and produce anextraintestinal amebiasis. In other words, E. histolytica is a facultative pathogen that exhibits a wide range of virulence.
The non-invasive disease is often asymptomatic, but can cause diarrhea or other gastro-intestinal symptoms such as abdominal pain or cramps. This non-invasive infection can persist or progress to an invasive disease in which trophozoites penetrate the intestinal mucosa and kill the epithelial cells. The early lesion is a small area of necrosis, or ulcer, characterized by raised edges and virtually no inflammation between lesions (Figure). The ameba will spread laterally and downward in the submucosa (beneath the epithelium) and kill host cells as they progress. This results in the classic 'flask-shaped' ulcer with a small opening and a wide base. Trophozoites are most numerous at the boundary between the healthy tissue and the necrotic tissue. These invasive ameba are ingesting host cells and trophozoites with ingested erythrocytes are often evident. These hematophagous trophozoites are sometimes found in the dysenteric feces. Cyst production decreases during the invasive stage of the infection and cysts are never found in the tissue lesions.
|
Left: The lumenal side of the colon from fulminating amebiasis case showing several ulcers. Note raised edges (arrow). Middle: Histological preparation showing cross-section of ulcer. Note the high degree of necrosis in center of ulcer. The amebas are advancing laterally under the intact mucosa as indicated by the microvilli. Right: Higher magnification of ulcer showing several hematophagous trophozoites. The nucleus (arrow) is evident in one of the amebas. Pictures from Peters and Gilles (1989), A Colour Atlas of Tropical Medicine and Parasitology (3rd edition). |
||
 |
|
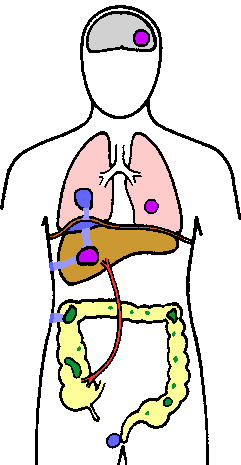
E. histolytica is found primarily in the colon where it can live as a non-pathogenic commensal or invade the intestinal mucosa (green). The ameba can metastasize to other organs via a hematogenous route (purple); primarily involving the portal vein and liver. The ameba can also spread via a direct expansion (blue) causing a pulmonary infection, cutaneous lesions or perianal ulcers. |
The ulcerative process may continue to expand laterally or downward. If large numbers of ulcers are present, they may coalesce which could lead to a localized sloughing off of the intestinal wall. Ulcer expansion can also penetrate the serous layer and lead to perforation of the intestinal wall. This perforation can lead to local abscesses or a generalized peritonitis. (See also schematic representation of tissue invasion.) Amebic ulcers can also become secondarily infected with bacteria which may confuse the clinical picture. In addition, E. histolytica infection can occasionally lead to the formation of an amebic granuloma, also called an ameboma. The ameboma is an inflammatory thickening of the intestinal wall around the ulcer which can be confused with a tumor.
Amebiasis can also progress to a systemic, or extraintestinal infection. Dissemination from the primary intestinal lesion is predominantly via the blood stream, but can also occur by direct extension of the lesion. The liver is the most commonly affected organ and this is probably due to the direct transport of trophozoites from the large intestine to the liver via the hepatic portal vein (Figure). Initially the lesions are small foci of necrosis which tend to coalesce into a single abscess as they expand. This hepatic abscess will continue to enlarge as the trophozoites progressively destroy and ingest host cells. The center of the abscess, consisting of lysed hepatocytes, erythrocytes, bile and fat, may liquefy and this necrotic material (sometimes incorrectly called pus) will range in color from yellowish to reddish brown. Secondary bacterial infections in the liver abscess are not common (~2%).
Hematogenous spread of trophozoites to other sites, such as the lungs or brain, is rare, but does occur. The second most common extraintestinal site after the liver is the lungs. Pulmonary infections generally result from a direct extension of the hepatic lesion across the diaphragm and into the pleura and lungs. Cutaneous lesions formed as a result of hepatic or intestinal fistula can also occur, although extremely rare. Other cutaneous lesions include perianal ulcers and involvement of the genitalia, including the penis of homosexuals. These later manifestations are likely due to the skin or mucous membranes coming in contact with invasive trophozoites.
 |
 |
 |
 |
| Entamoeba Prevalences |
|
As discussed above, E. histolytica is pathogen that exhibits a wide spectrum of virulence, ranging from an avirulent commensal to a highly invasive and destructive organism (see discussion of pathogenicity vs. virulence). Some of this difference in virulence is explained by the existence of the morphologically identical, but avirulent, E. dispar. E. dispar has never been associated with a symptomatic invasive disease and infection does not elicit serum antibodies. In contrast, anti-ameba humoral responses are observed in both asymptomatic and symptomatic E. histolytica infections. This suggests that even in asymptomatic cases there is a limited amount of invasion. However, infection with E. histolytica does not always lead to invasive disease, though, in that only about 10% of the infected individuals will develop symptomatic invasive amebiasis. The factors responsible for the pathogenesis of E. histolytica are not well understood. One approach to understanding the pathogenesis is to compare possible virulence factors between these two closely related species.
| host factors
parasite factors
|
Pathology results from host-parasite interactions, and therefore, host factors, parasite factors or a combination of both may contribute to the disease state. For example, the development of invasive disease could be due to quantitative or qualitative aspects of the host immune response. Recruitment of neutrophils and intense inflammation are noted in the early phases of amebic invasion. However, inflammation surrounding established ulcers and abscesses if often minimal given the degree of tissue damage.
The nature of protective immune responses is not clear. Innate or nonspecific immunity, as well as acquired immunity, are probably both important for the prevention of invasive disease. The mucous layer covering the epitheilial cells can prevent contact between trophozoite and host cells. In addition, mucosal IgA responses do occur as a result of infection and fecal IgA against a trophozoite surface lectin (see Eh-lectin) are associated with a lower incidence of new E. histolytica infections. High titers of serum antibodies also develop in patients with liver abscesses. However, since the invasive disease is often progressive and unremitting, the role of these anti-ameba antibodies is in question. Cell-mediated responses appear to play a role in limiting the extent of invasive amebiasis and protecting the host from recurrence following successful treatment.
Resistance to the host immune response is another possible virulence factor which could contribute to the development and exacerbation of invasive disease. For example, one phenotypic difference between E. dispar and E. histolytica is the resistance of the latter to complement mediated lysis (see E. dispar). In addition, E. histolytica rapidly degrades secretory IgA and possibly suppresses T-cell responses to E. histolytica antigens. E. histolytica is also able to kill cells, including neutrophils and other immune effector cells, in a contact dependent manner. Lysis of neutrophils could also release toxic products which contribute to the destruction of host tissue. However, the role of these various phenomena in pathogenesis is not known.
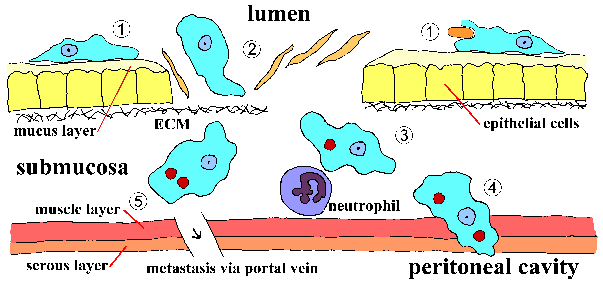
Invasion of intestinal mucosa by E. histolytica is an active process mediated by the parasite and distinct steps can be recognized (Figure, click here for larger image and detailed legend). Trophozoites adhere to the mucus layer (step 1). This adherence per se probably does not contribute to pathogenesis and is simply a mechanism for the ameba to crawl along the substratum. Depletion of the mucus barrier allows for the trophozoite to come in contact with epithelial cells. Epithelial cells are killed in a contact dependent manner leading to a disruption of the intestinal mucosa (step 2). The trophozoites will continue to kill host cells in the submucosa and further disrupt the tissue as they advance (step 3). Disruption of the intestinal wall (step 4) or metastasis via the circulatory system (step 5) is also possible. Adherence, cytotoxicity, and disruption of the tissues are important factors in the pathogenesis of E. histolytica. Parasite proteins which could play a role in these processes include: the Eh-lectin, amebapore, and proteases.
(Skip detailed discussions of Eh-lectin, amebapore, and proteases and go to clinical symptoms.)
Eh-lectin. E. histolytica can kill cells within minutes of adhering to them in the presence of extracellular calcium. Adherence of E. histolytica trophozoites to host cells and colonic mucins is mediated by a lectin-activity expressed on the ameba's surface. This lectin binds galactose or N-acetyl-D-galactosamine (GalNAc) with a high affinity and is also called the galactose-inhibitable adherence protein (GIAP) or the Gal/GalNAc lectin. The contact-dependent killing of target cells is almost completely inhibited by galactose or GalNAc and target cells lacking terminal galactose residues on their surface glycoproteins are resistant to trophozoite adherence and cytotoxicity. This suggests that the Gal/GalNAc lectin is an important virulence factor. In addition, the Eh-lectin is involved in resistance to complement mediated lysis. Because of its potential role in adherence and virulence and since fecal IgA against it protect against amebic colitis, the Gal/GalNAc is a vaccine candidate (Petri et al, 2006, Arch. Med. Res. 37:288).
The Eh-lectin is a heterodimer consisting of a 170 kDa heavy chain and a 31-35 kDa light chain joined by disulfide bonds. An intermediate subunit of 150 kDa is noncovalently associated with the heterodimer. The heavy chain has a transmembrane domain and a carbohydrate binding domain. All of subunits are encoded by multigene families. There are five members of the heavy chain family, 6-7 members of the light chain family and 30 members of the intermediate chain family. The members of the heavy chain gene family exhibit 89-95% sequence identity at the amino acid level whereas the light chain family members are less conserved sharing only 79-85% sequence identity.
E. dispar also expresses Gal/GalNAc lectin on its surface. Both E. dispar and E. histolytica need to adhere to the mucous layer which is medicated by the Gal/GalNAc lectin. Mucus is composed of glycoproteins called mucins. The predominant mucin found on the intestinal mucosa is Muc2 which is extensively glycosylated with O-linked GalNAc residues. The sequence of the light and heavy chain genes from E. dispar are homologous, but not identical, to those of E. histolytica. Antigenic differences between the GIAP of E. dispar and E. histolytica have also been described in that only two epitopes out of six are shared between the two species (see E. dispar). It is not known whether these sequence differences can account for the differences in virulence between E. dispar and E. histolytica. Adherence is obviously important for both species, but it is possible that the adherence is qualitatively or quantitatively different between the two species.
[Review on the Eh-lectin: Petri et al (2002) Annu. Rev. Microbiol. 56:39.]
Amebapore. A family of pore-forming polypeptides has been identified in E. histolytica and E. dispar. The three family members are designated as amebapore A, B and C with amebapore A being predominant expressed. The mature polypeptide is 77 amino acids long and forms dimers at low pH (4-6). Three of these dimers then assemble into a hollow ring-shaped structure. This hexamer then can intercalate into membranes and introduce 2 nm pores (i.e., holes) which results in cell death. The pore-forming activity is dependent on this assembly process beginning with the dimerization. Amebabpore A is 95% identical (i.e., four residues are different) between E. histolytica and E. dispar. In addition, the E. dispar amebapore has approximately half of the pore-forming activity as the E. histolytica amebapore. This difference in pore-forming activity has been attributed to a glutamate residue at position 2 in the E. histolytica amebapore, as compared to a proline residue in the E. dispar amebapore. This particular amino acid residue is important for the formation of the dimers and it is believed that the dimers of E. dispar amebapore are less stable.
Amebapore is localized to vacuolar compartments (eg, food vacuoles) within the trophozoite and is most active at acidic pH suggesting that the major function of amebapore is to lyse ingested bacteria. Nonetheless, amebapore is implicated as a virulence factor in that genetic manipulation of E. histolytica resulting in decreased expression of amebapore leads to a reduction in pathogenicity (ability to form liver abscesses) as well as a reduction in bacteriocidal activity (Bracha et al Mol. Microbiol. 34:363, 1999). Similarly, modified E. histolytica completely devoid of amebapore production are unable to form liver abscesses in model systems (Zhang et al, Inf. Imm. 72:678, 2004). However, these amebas are able to cause inflammation and tissue damage in models for amebic colitis.
[Review on amebapore: Leippe et al, Tr. Parasitol. 21:5, 2005.]Proteases. Proteases are enzymes that degrade other proteins and could contribute to the pathogenesis cause by E. histolytica. In this regard, E. histolytica expresses and secretes higher levels of cysteine proteases, a particular class of protease, than E. dispar. Cysteine proteases have been shown to disrupt the polymerization of MUC2, the major component of colonic mucus. This degraded mucus is less efficient at blocking adherence of trophozoites to epithelial cells. Destruction of the extracellular matrix (ECM) by proteases may also facilitate trophozoite invasion. Inhibitors of cysteine proteases can decrease liver abscess size in experimental models.
|
|
|
|
Factor |
histolytica vs dispar |
| Eh-lectin | sequence and epitope differences |
| amebapore | Ed has less activity (Pro/Glu) |
| proteases | Eh has unique genes and expresses more activity |
| Figure from Horstmann et al (1992) Trop. Med. Parasitol. 43, 213. | |
Twenty different cysteine protease genes have been identified in E. histolytica. Orthologs of two of the E. histolytica cysteine protease genes are not found in E. dispar. One of these, designated CP5, is expressed at high levels on the trophozoite surface. Mutants expressing lower levels of CP5 had a reduced ability to generate liver abscesses in a hamster amebiasis model. However, these mutants also had a reduced growth rate and lower erythrophagocytic activity, thus it is not clear whether CP5 directly participates in the invasiveness of E. histolytica. Furthermore inhibition of 90% of CP5 activity did not affect the ability of E. histolytica trophozoites to destroy cell monolayers in vitro. CP1, CP2, and CP5 are the most abundantly expressed cysteine proteases in E. histolytica, whereas CP3 is the most abundant in E. dispar. Interestingly, over expression of CP2 in E. dispar increased the ability of trophozoites to destroy cell monolayers in vitro. However, the over expression of CP2 did not lead to the ability of E. dispar to form liver abscesses in gerbils. Therefore, it is not clear the precise roles proteases may play in pathogenesis.
In summary, the pathogenesis associated with E. histolytica infection is primarily due to its ability to invade tissues and kill host cells. Several potential virulence factors have been identified (see Table). However, it is not clear the exact role these various virulence factors play in the development of invasive disease. One approach to understanding the pathogenesis is to compare these factors from E. histolytica and E. dispar. These two species are closely related and the potential virulence factors are found in both species. Adherence, cytolytic activity and proteolytic activity are inherent biological features of both species and these activities do not necessarily lead to pathology. However, there are qualitative and quantitative differences between E. histolytica and E. dispar which may account for the differences in virulence. These genetic differences between E. histolytica and E. dispar indicate that pathogenesis is in part an inherent feature of the parasite. However, pathogenesis is probably due to the combined effects of several host and parasite factors, and the virulence may represent the degree to which the host can control trophozoite invasion and replication.
[See Huston, 2004, Tr. Parasitol. 20:23 or Ralston and Petri, 2011, Tr. Parasitol. 27:253 for reviews on pathogenesis.]
 |
 |
 |
 |
Amebiasis presents a wide range of clinical syndromes (Table) which reflects the potential for E. histolytica to become invasive and cause a progressive disease. The incubation period can range from a few days to months or years with 2-4 weeks being the most common. Transitions from one type of intestinal syndrome to another can occur and intestinal infections can give rise to extraintestinal infections.
Intestinal
Disease
|
The majority of individuals diagnosed with E. histolytica (or E. dispar) exhibit no symptoms or have vague and nonspecific abdominal symptoms. This state can persist or progress to a symptomatic infection. Symptomatic nondysenteric infections exhibit variable symptoms ranging from mild and transient to intense and long lasting. Typical symptoms include: diarrhea, cramps, flatulence, nausea, and anorexia. The diarrhea frequently alternates with periods of constipation or soft stools. Stools sometimes contain mucus, but there is no visible blood.
Amebic dysentery usually starts slowly over several days with abdominal cramps, tenesmus, and occassional loose stools, but progresses to diarrhea with blood and mucus. Blood, mucus and pieces of necrotic tissue become more evident as the number of stools increases (10-20 or more per day) and stools will often contain little fecal material. A few patients may develop fever, vomiting, abdominal tenderness, or dehydration (especially children) as the severity of the disease increases. Fulminant, or grangrenous, colitus is a rare but extremely severe form of intestinal amebiasis. Patients present with severe bloody diarrhea, fever, and diffuse abdominal tenderness. Most of the mucosa is involved and mortality exceeds 50%. A chronic amebiasis, characterized by recurrent attacks of dysentery with intervening periods of mild or moderate gastrointestinal symptoms, can also occur.
Amebomas present as painful abdominal masses which occur most frequently in the cecum and ascending colon. Obstructive symptoms or hemorrhages may also be associated with an ameboma. Amebomas are infrequent and can be confused with carcinomas or tumors. Perianal ulcers are a form of cutaneous amebiasis that result from the direct spread of the intestinal infection.
Amebic liver abscesses are the most common form of extraintestinal amebiasis. The onset of hepatic symptoms can be rapid or gradual. Hepatic infections are characterized by hepatomegaly, liver tenderness, pain in the upper right quadrant, fever and anorexia. Fever sometimes occurs on a daily basis in the afternoon or evening. Liver function tests are usually normal or slightly abnormal and jaundice is unusual. Liver abscesses will occasionally rupture into the peritoneum resulting in peritonitis.
Pulmonary amebiasis is generally results from the direct extension of the liver abscess through the diaphragm. Clinical symptoms most often include cough, chest pain, dyspnea (difficult breathing), and fever. The sputum may be purulent or blood-stained and contain trophozoites. A profuse expectoration (ie, vomica) of purulent material can also occur. Primary metastasis to the lungs is rare, but does occur. Similarly, infection of other organs (eg., brain, spleen, pericardium) is also rare. Clinical symptoms are related to the affected organ.
Cutaneous amebiasis is the result of skin or mucus membranes being bathed in fluids containing trophozoites. This contact can be the result of fistula (intestinal, hepatic, perineal) or an invasion of the genitalia. Cutaneous lesions have a wet, granular, necrotic surface with prominent borders and can be highly destructive. Clinical diagnosis is difficult and is usually considered with epidemiological risk factors (eg., endemic areas, male homosexuality, etc.).
 |
 |
 |
 |
| Diagnosis |
Intestinal
Disease
|
Definitive diagnosis of amebiasis requires the demonstration of E. histolytica cysts or trophozoites in feces or tissues. Stool specimens should be preserved and stained and microscopically examined. Cysts will tend to predominate in formed stools and trophozoites in diarrheic stools (see morphology). Fresh stools can also be immediately examined for motile trophozoites which exhibit a progressive motility. Sigmoidoscopy may reveal the characteristic ulcers, especially in more severe disease. Aspirates or biopsies should also be examined microscopically for trophozoites.
E. histolytica and E. dispar cannot be distinguished on morphological criteria. Antigen detection kits are available for the positive identification of these species. One such rapid antigen detection test is the E. HISTOLYTICA QUIK CHEK (TechLab, Inc).
Serology is especially useful for the diagnosis of extraintestinal amebiasis. Greater than 90% of patients with invasive colitis and liver abscesses exhibit serum antibodies against E. histolytica. However, the antibodies can persist for years and distinguishing past and current infections may pose problems in endemic areas. Non-invasive imaging techniques (eg., ultrasound, CT, MRI) can be used to detect hepatic abscesses. It is also possible to aspirate hepatic abscesses. However, this is rarely done and only indicated in selected cases (eg., serology and imaging not available, therapeutic purposes). The aspirate is usually a thick reddish brown liquid that rarely contains trophozoites. Trophozoites are most likely to be found at the abscess wall and not in the necrotic debris at the abscess center.
Several drugs are available for the treatment of amebiasis and the choice of drug(s) depends on the clinical stage of the infection (Table). The prognosis following treatment is generally good in uncomplicated cases. In cases where E. histolytica is confirmed or the species (ie, dispar or histolytica) is unknown, asymptomatic cyst passers should be treated to prevent the progression to severe disease and to control the spread of the disease. However, in many endemic areas, where the rates of reinfection are high and treatment is expensive, the standard practice is to only treat symptomatic cases. Metronidazole or tinidazole (if available) is recommended for all symptomatic infections. This treatment should be followed by or combined with lumenal antiamebic drugs as described for asymptomatic patients.
Amebiasis Treatment |
||||||||
|
In the cases of fulminant amemic colitis or perforation of the intestinal wall a broad spectrum antibiotic can also be used to treat intestinal bacteria in the peritoneum. Necrotic colitis requires urgent hospitalization to restore fluid and electrolyte balance. In addition, emetine or dehydroemetine are sometimes co-administered with the nitroimidazole. This is only done in the most severe cases due to the toxicity of these drugs. Surgery may also be needed to close perforations or a partial colostomy. Abscess drainage of hepatic lesions (ie, needle aspiration or surgical drainage) is now rarely performed for therapeutic purposes and is only indicated in cases of large abscesses with a high probability of rupture.
Prevention and control measures are similar to other diseases transmitted by the fecal-oral route (see Risk Factors or discussion of Giardia control). The major difference is that humans are the only host for E. histolytica and there is no possibility of zoonotic transmission. Control is based on avoiding the contamination of food or water with fecal material. Health education in regards to improving personal hygiene, sanitary disposal of feces, and hand washing are particularly effective. Protecting water supplies will lower endemicity and epidemics. Like Giardia, Entamoeba cysts are resistant to standard chlorine treatment, but are killed by iodine or boiling. Sedimentation and filtration processes are quite effective at removing Entamoeba cysts. Chemoprophylaxis is not recommended.
Reviews on amebiasis:
Blastocystis hominis is a common organism found
in human stools. Since its initial description approximately 100 years ago,
it has been variously classified as an ameba, a yeast, a sporozoan, and the
cyst stage of a flagellate. Analysis of the small subunit rRNA sequence indicates
that Blastocystis is most closely related to the stramenopiles, a complex
assemblage of unicellular and muticellular protists. Other stramenopiles include
diatoms, brown algae, and water molds. Many of the characteristics of Blastocystis
are unknown or controversial. The mode of transmission, mechanism of cell replication,
and other features of the life cycle have not conclusively demonstrated. Similarly,
the status of Blastocystis as a pathogen, commensal, or opportunistic
organism is unknown.
Blastocystis is polymorphic in that a variety of morphological forms are found in feces and in vitro culture. The most widely recognized form is spherical 10-15 Ám in diameter with a large central vacuole (Figure). This large vacuole pushes the nuclei and other organelles to the periphery of the cell. The vacuole is sometimes filled with a granular material. Small resistant cyst-like forms have been identified from in vitro cultures and occasionally observed in feces. These presumed cysts are approximately 5 Ám and surround by a multilayered wall. Furthermore, the cysts do not lyse when placed in water suggesting that they are resistant to environmental conditions. Presumably Blastocystis is transmitted via a fecal-oral route. However, this has not been conclusively demonstrated.
There have been several reports suggesting Blastocystis causes disease, as well as many reports suggesting the opposite. Diarrhea, cramps, nausea, vomiting and abdominal pain have been associated with large numbers of organisms in the stool. In addition, some studies have shown that treatment alleviates the symptoms and clears the organisms. However, the drugs used against Blastocystis (eg., metronidazole) also work against many other intestinal protozoa and bacteria. The inability to rule out other organisms as the source of symptoms and the observation that many infected persons exhibit no symptoms makes it difficult to draw any definitive conclusions about the pathogenesis of Blastocystis. Furthermore, it could be that Blastocystis is primarily a commensal, but can exhibit virulence under specific host conditions like concomitant infections, poor nutrition, or immunosuppression.
Blastocystis is also found in a wide range of animals, including mammals, birds, reptiles, amphibians and even insects, and exhibits a wide range of molecular diversity. The genetic distance between Blastocystis isolates is greater than the genetic distance between E. histolytica and E. dispar (see discussion on E. dispar). This complicates the designation of species and historically human isolates have been designated as B. hominis and isolates for other hosts as Blastocytis sp.. However, phylogenetic analysis reveals that there are no exclusively human clades and human isolates are found in all of the clades. This raises the possibility that Blastocystis is not host specific and can be transmitted zoonotically. In addition, the wide range of genetic diversity might explain the controversy concerning the pathogenecity of Blastocystis in that some genotypes may be more virulent than others. However, studies addressing this issue suggest that this is not the case. Resolution of the confusion about the taxonomy, transmission and virulence of Blastocystis will require additional studies.
Reviews on Blastocystis:
 |
 |
 |
 |
Numerous protozoa can inhabit the gastro-intestinal tract of humans. Most of these exhibit little or no overt pathology. Infection with these protozoa is evidence of fecal contamination and indicates a risk for more serious infections such as Giardia or E. histolytica. These non-pathogenic species can also be confused with the potentially pathogenic Giardia or E. histolytica and result in unnecessary drug treatment. In addition, such a misdiagnosis is also problematic in that the true cause of the symptoms may be missed and the appropriate treatment will be delayed.
|
Several Entamoeba species infect humans (box). E. histolytica can cause a severe intestinal disease characterized by dysentery as well as an invasive disease affecting primarily the liver (see Amebiasis). E. dispar is morphologically identical to E. histolytica, but does not produce an invasive disease (see further discussion on E. dispar). A distinguishing feature of the Entamoeba is their nuclear morphology which is described as having peripheral chromatin and a small karyosome. E. histolytica/dispar, E.coli, and E. hartmanni can be distinguished by size and minor morphological differences (see Table).
| Intestinal Entamoeba Species | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli is the largest and is best distinguished by 8 nuclei in the mature cyst. The trophozoites of E. coli can be difficult to distinguish from E. histolytica/dispar since there is some overlap in the size ranges. E. hartmanni is quite similar to E. histolytica and was previously considered a 'small race' of E. histolytica. Generally 10 Ám is chosen as the boundary between E. histolytica and E. hartmanni.
E. polecki is usually associated with pigs and monkeys, but human cases have been occasionally documented. It appears to be geographically restricted to particular areas such a Papua, New Guinea. The trophozoites are similar to E. coli, except a little smaller, and the cysts are similar to E. histolytica except that the mature cyst has a single nucleus.
E. gingivalis can be recovered from the soft tartar between teeth and exhibits a similar morphology to E. histolytica except that it has no cyst stage. E. gingivalis can also multiply in bronchial mucus, and thus can appear in the sputum. In this case it could be confused with E. histolytica from a pulmonary abscess. E. gingivalis trophozoites will often contain ingested leukocytes which can be used to differentiate it from E. histolytica. The trophozoites are most often recovered from patients with periodontal disease, but an etiology between the organism and disease has not been established and E. gingivalis is considered to be non-pathogenic.
Other non-pathogenic amebae include Endolimax nana and Iodoamoeba bütschlii. Historically, Dientamoeba fragilis has been grouped with the ameba, but electron microscopy and molecular phylogenetics suggests that it is actually a flagellate and may be closely related to the trichomonads (see above). All three of these organisms exhibit similar morphologies and have nuclei which do not have peripheral chromatin and a large karyosome. Minor morphological differences allow these organims to be distinguished (Table).
| Other Intestinal Amebae | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Four additional non-pathogenic flagellates recovered from human stools are: Trichomonas hominis, Chilomastix mesnili, Enteromonas hominis, and Retortamonas intestinalis. Among these T. hominis, also called Pentatrichomonas hominis, is the most common and is often recovered from diarrheic stools. These flagellates exhibit similar morphologies (Table) and can be difficult to distinguish. The trophozoites from all of these flagellates are somewhat teardrop shaped and contain a single nucleus and the cyst tend to be slightly elongated or oval.
| Other Intestinal Flagellates | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||
These pages are developed and maintained by Mark F. Wiser, Tulane University (©2000). Last update on June 24, 2021 .