The Biomechanics of Growth & Remodeling Laboratory uses a combined experimental and computational approach to better understand, describe, and predict soft tissue remodeling in response to various biochemomechanical stimuli including normal processes (e.g., aging and pregnancy), disease, and injury. To this end, our research utilizes model systems with varying restraints on regenerative capability (postnatal development, pregnancy, postpartum, and aging) as well as genetically modified animals. We utilize sophisticated mechanical testing techniques and computational growth and remodeling models to elucidate dynamic structure-function relationships in evolving collagenous tissues in both maintenance (homeostatic) states and maladaptive remodeling (e.g., fibrosis, degeneration). In particular, we are interested in identifying the appropriate biochemomechanical stimuli and matrix components necessary for proper collagen fibrillogenesis and elastogenesis in adult tissues. Further, experiments and computational models are used to identify potential treatments and the appropriate timecourse for clinical interventions to prevent maladaptive remodeling, improve adult response to injury, and advance tissue engineering strategies. Our primary areas of research include women’s reproductive health, wound healing, and orthopaedics (tendon and ligament).
Women's Reproductive Health
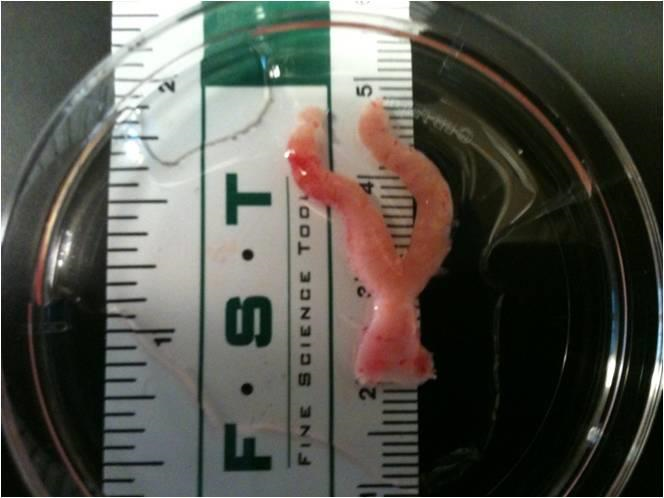
The goal of this area of study is to gain a better understanding of the relationship between reproductive tissue extracellular matrix constituents and mechanical properties of the vagina, cervix, and uterus. These studies will evaluate the contribution of collagen, elastin, smooth muscle, and glycosaminoglycans to reproductive tissue function. The results of this study will enable scientists to better understand the relationship between structure and function (mechanical properties: strength) of the reproductive system to gain valuable insights toward understanding remodeling during pregnancy, postpartum recovery, and aging to identify potential causes of cervical insufficiency and pelvic organ prolapse. These studies will help design treatments to prevent premature birth and pelvic organ prolapse and improve the clinical treatment of cervical insufficiency.
Dynamic Cervical Mechanics
During pregnancy and postpartum, the cervix is subjected to various mechanical stimuli (growing fetus) and chemical stimuli (e.g, inflammation and hormones) which dictate rapid ECM remodeling, including changes in collagen network structure and cervical compliance. A complex series of biochemomechanical perturbations dictate cervical remodeling throughout the softening, ripening, and dilation phases of pregnancy as the cervix must initially remain closed to maintain the growing fetus, and the open sufficiently to allow passage at term. Following labor and delivery, the post-partum repair process begins and rapidly restores cervical mechanical and microstructural integrity. To this end, the our lab utilizes the cervix as model system to better understand, describe, and predict ECM remodeling in response to various chemomechanical stimuli. In particular, we seek to unravel the complex interaction between mechanical and biochemical (e.g., inflammatory and hormonal) stimuli that dictate dynamic tissue remodeling, including the potential roles of multiple types and phenotypes of matrix producing cells (both on matrix production and subsequent remodeling) as well as the complex interactions between load-bearing matrix constituents (e.g., collagen integrity and glycosaminoglycans). Collaborators: Leise Kneopp MD (Ochsner Baptist)
Dynamic Vaginal Mechanics
This area of research is dedicated to elucidating the underlying cause of pelvic organ prolapse (POP), a global health concern characterized by the atrophy of the vaginal wall and the downward decent of the pelvic organs. Little is known about the underlying pathology, but there is a well-established connection between POP and connective tissue metabolism. Therefore, the ability to accurately quantify the mechanical properties of murine vaginal tissue and the role of the load bearing constituents under normal homeostatic conditions as well as in diseased states is essential to elucidating the underlying pathology. To this end our lab utilizes the murine vagina as a model system and physiologically relevant multi-axial biomechanical testing protocols to rigorously quantify the dynamic structure-function relationships of the vagina during the healthy maintenance state and to elucidate the complex interactions between the load bearing constituents.
Orthopaedics - Tendon & Ligament Homeostasis and Degenerative Microtrauma
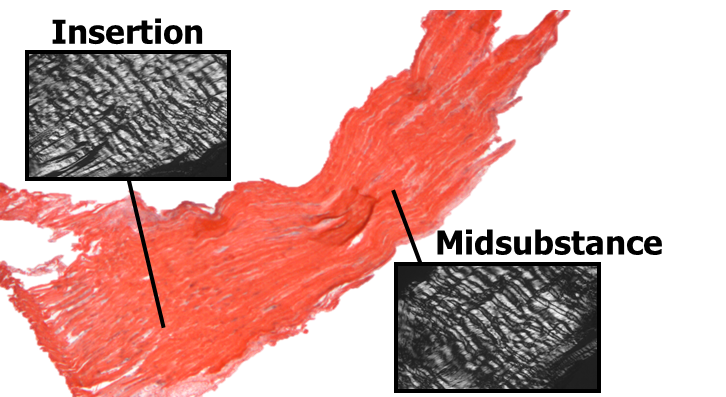
The primary load-bearing constituent of tendon and ligament is type I collagen. Hence, tendons & ligaments are excellent model systems to examine the production and removal of fibrillar collagen and small leucine rich proteoglycans (SLRPs) in maintenance (homeostasis) conditions as well as instances of maladaptive or inadequate remodeling. The basal production and half-life of collagen, however, is not clearly defined for tendon & ligament and is thought to depend on the tissue location and physiologic function. Hence, there is a pressing need to better understand the basal production, removal, and post-translational modifications of collagen in tendon maintenance (homeostasis). In particular, we seek to understand the ability of cells to respond to variations in mechanical stimuli through the production, removal, and remodeling of collagen and other structurally significant ECM proteins (e.g., elastin and GAG/PGs).
Tendon & Ligament Structure-Function Relationships
The overall purpose of this area of research is to investigate the relationships between the structurally-significant constituents, such as type I fibrillar collagen, of tendons and ligaments and their corresponding physiologic function. These studies are necessary to identify the role of structurally-significant constituents in dynamic connective tissue homeostasis, injury, and repair. In addition, identification of structure-function relationships for connective tissues can provide guidance towards the design of patient-specific clinical interventions and improved tissue engineering strategies.
Tissue Engineering and Regenerative Medicine
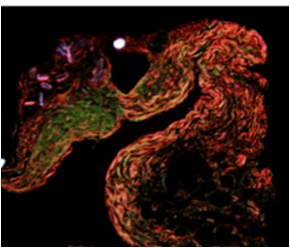
The field of tissue engineering has rapid advanced in recent years, including advances in fabrication techniques such as electrospinning and 3-D printing. Given the myriad of possible combinations of scaffold parameters and materials, there is a need for a new strategy to reduce the experimental search space. Toward this end, we utilize hypothesis-driven computational G&R models to identify suggested scaffold parameters and to inform experiments to move towards the rational design of tissue engineered constructs. Further, we utilize in vivo and in vitro model systems to better understand the role of varying types (and phenotypes) of matrix producing cells, including examining the role of cell interactions on matrix production and subsequent remodeling. Collaborators: Jay D. Humphrey PhD (Yale University), Christopher K. Breuer MD (Nationwide Children's Hospital), Alison Marsden (UCSD)
